Method for improving cellulase expression of trichoderma reesei by interfering phosphatase gene
A phosphatase gene, technology of Trichoderma reesei, applied in the field of genetic engineering, can solve the problems of decreased secretion of RutC-30 protein, decreased cellulase activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
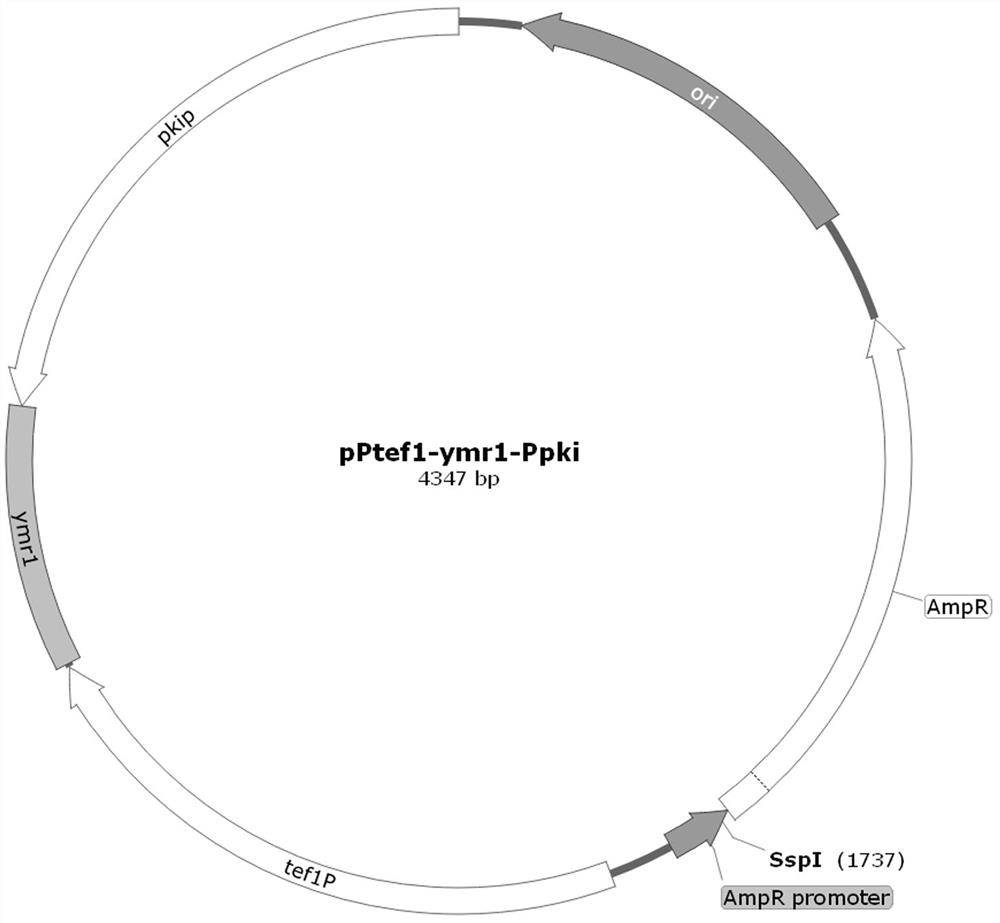
[0016] Example 1 Construction of pPtef1-ymr1-Ppki plasmid
[0017] Construction of the pPtef1-ymr1-Ppki plasmid: tef1 Promoter, ymr1 Interference gene 413 bp, pki Promoter and ampr+ori sections. Using the APA-GOD plasmid as a template, amplify the ampr+ori part; using the Trichoderma reesei genome as a template, tef1 Promoter, ymr1 Interference gene 413 bp (ymr1iF: GACAATGACCAGCAGACCATCGAG
[0018] ;ymr1iR:CTTTTCGCTCTGATAATGGCCAGG) and pki Promoter, electrophoresis, and 4 recovered fragments were connected by homologous recombination. Transform Escherichia coli Trans1-T1 competent cells, perform colony PCR on the colonies grown on the plate, send the colonies identified as positive by PCR to sequencing, and name the plasmid with correct sequencing as pPtef1-ymr1-Ppki plasmid, such as figure 1 .
Embodiment 2
[0019] Example 2. Introduction of pPtef1-ymr1-Ppki plasmid
[0020] (1) Transform Trichoderma reesei SUS4-1 with pPtef1-ymr1-Ppki plasmid
[0021] Trichoderma reesei SUS4-1 was inoculated on a potato medium (PDA) plate, and cultured statically at 28 ºC for 7 days until it produced spores, and the spores were scraped off and inoculated in 100 mg / ml PDB medium containing uracil. Incubate overnight at 28ºC with shaking at 160 rpm. Collect the germinated mycelium by filtering through a 200-mesh sieve, add 10 mg / ml cellulase and digest at 30°C for 2-3 hours. After protoplasts were collected, the pPtef1-ymr1-Ppki plasmid was used Ssp I digestion, transformation of Trichoderma reesei host cells.
[0022] (2) PCR verification of the introduction of the pPtef1-ymr1-Ppki plasmid into the Trichoderma reesei genome
[0023] A single transformant was picked, inoculated on a 24-well plate containing MM-glucose medium, and cultured at 28°C for 5-7 days. Genomic DNA was extracted to ver...
Embodiment 3
[0024] Example 3. pPtef1-ymr1-Ppki Effect on protein expression
[0025] (1) interference ymr1 Shake flask induction of transformants
[0026] interference ymr1 Transformants and starting strains were inoculated with 2×10 7 The spores were cultured in 50 ml MM-glucose medium at 28°C and 160 rpm for 2 days. Transfer to 50 ml MM+2% Avicel medium with 10% inoculum size to induce the expression of cellulase. From the third day, samples were taken every 24 h, and the samples were taken continuously for 7 days.
[0027] (2) interference ymr1 Determination of the protein concentration and cellulase of the transformant of the gene
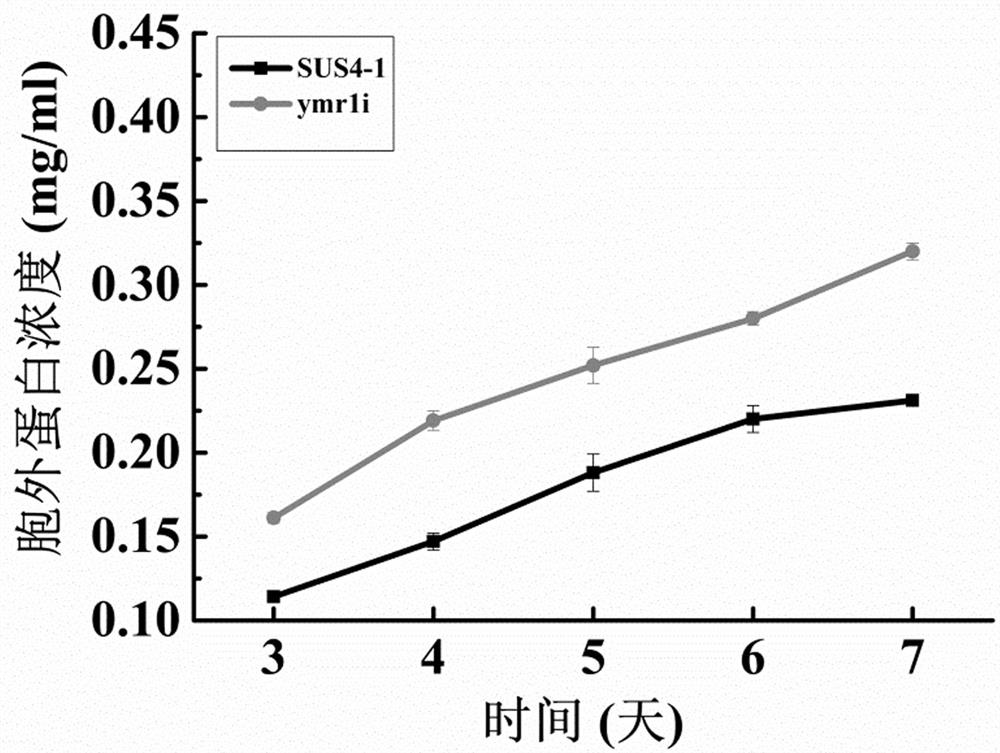
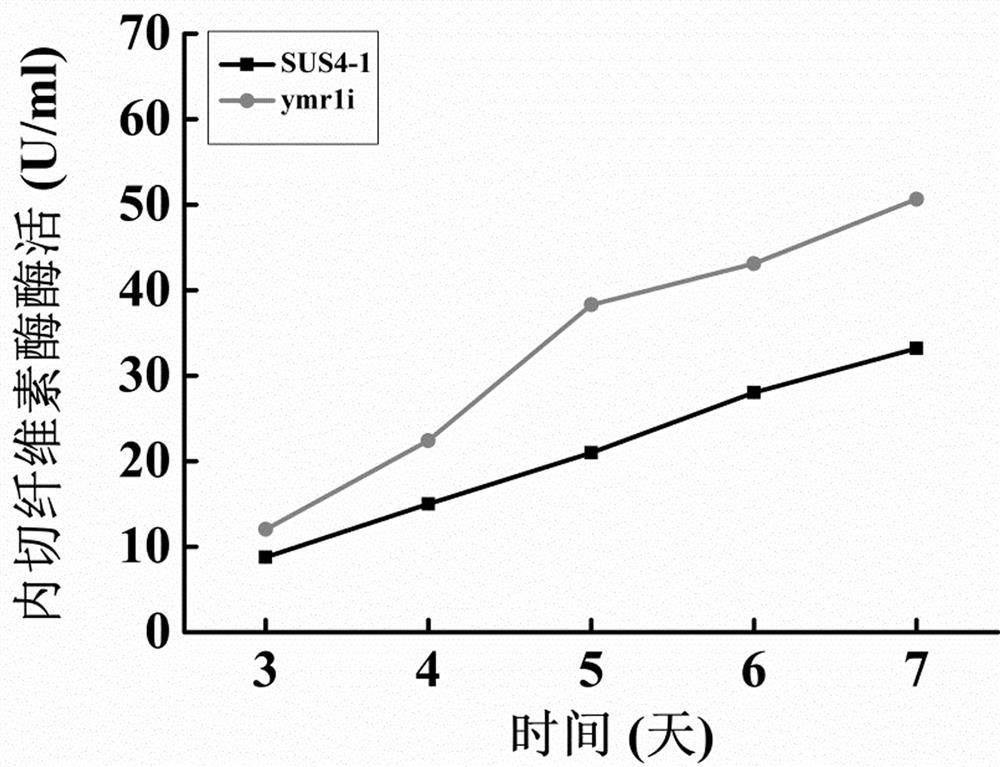
[0028] The protein was quantified by Coomassie Brilliant Blue method, after adding 250 µl 1 × dye reagent and 10 µl protein standard, reacted at room temperature for 10 minutes, then measured the absorbance at 595 nm, the results are shown in figure 2 . visible ymr1 The concentration of protein secreted extracellularly by the transformant a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com