Delayed disintegration-type capsule and method for producing same
A manufacturing method and disintegration technology, which can be used in microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve the problem of reducing the degree of freedom of film design, and achieve the effect of easy design and increased degree of freedom.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
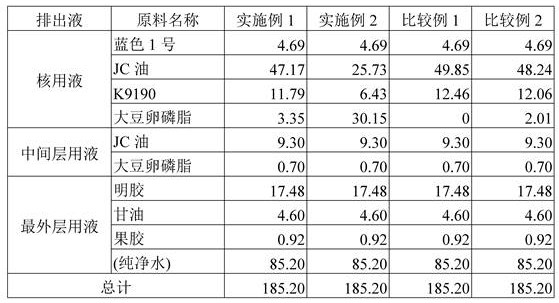
[0064] (a) Nuclear Liquid: Stirring 47.17 parts by weight of Hydrofin (JC oil made by solar oil: 52 ± 3 ° C), 11.79 parts by weight of H9190: melting point 49 ± 3 ° C) And 3.35 parts by weight of soy lecithin were uniformly dissolved, and 4.69 parts by weight of blue No. 1 was mixed in the resulting solubility to be used as a nuclei.
[0065] (b) Intermediate layer solution: mix 9.30 parts by weight of edible oil (JC oil manufactured by solar oil: 52 ± 3 ° C) and 0.7 parts by weight of soy lecithin as an intermediate layer solution.
[0066] (c) Older solution: mix 17.48 parts by weight of gelatin at 60 ° C [Jellystregth, gel strength): 280 Brony (Bloom)], 4.60 parts by weight of glycerol, 0.92 parts by weight of low methoxy Base (LM) pectin and 85.20 parts by weight of pure water, as the outermost liquid.
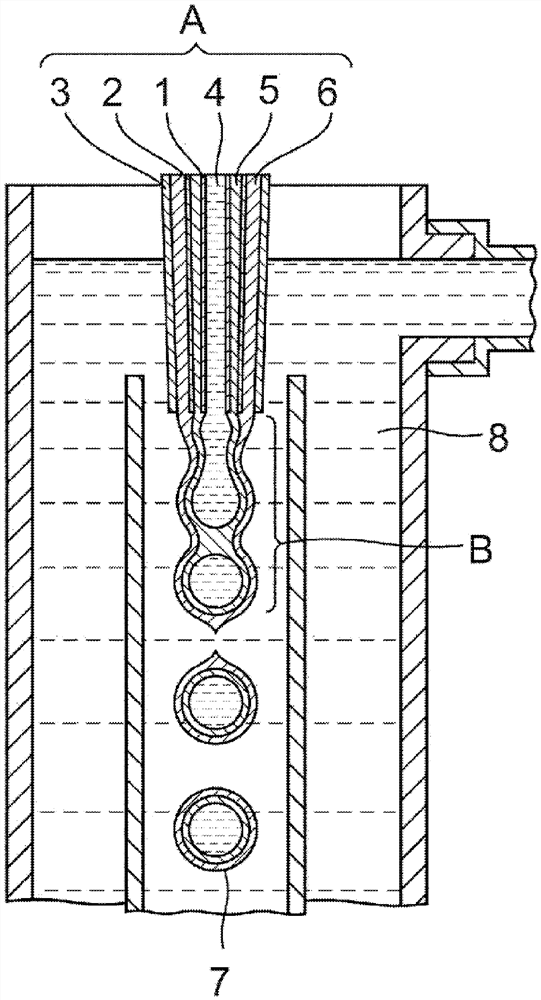
[0067]Triple nozzle concentrically with the innermost nozzle of the core with the solution was added dropwise, and the intermediate layer from the nozzle dropwise with mediola...
Embodiment 2 and comparative example 1~2
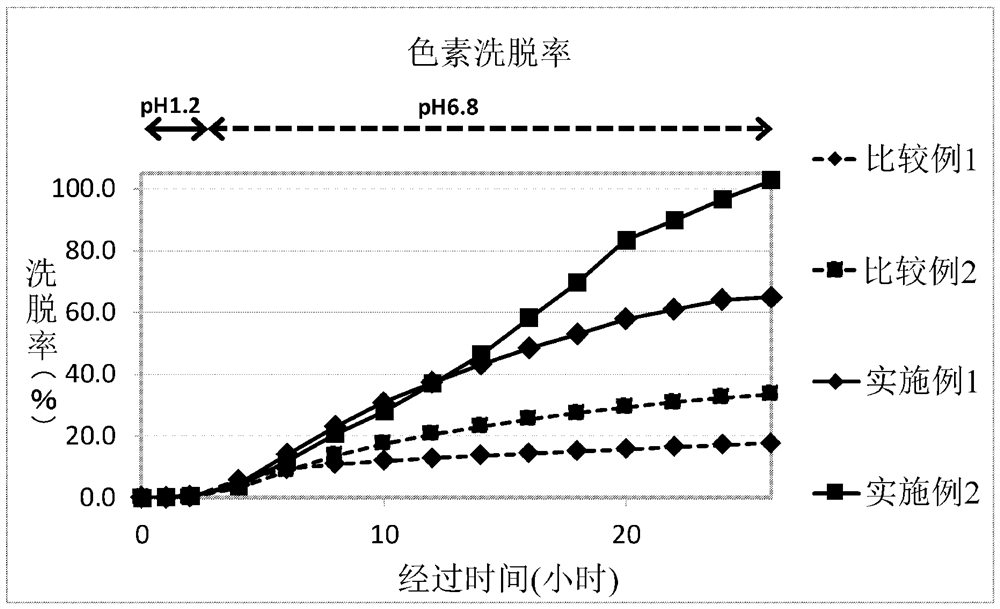
[0074] Using shown in the following Table 1 were blended in the same manner as in Example 1 formed seamless capsule, the elution experiments carried out in the same essentials. In Example 1, results are figure 2 .
[0075] [Table 1]
[0076]
[0077] The above Examples and Comparative Examples, and figure 2 It found: Examples and Comparative Examples were eluted from the start of the experiment started eluting after about 4 hours pigment, but after the start of the experiment eight hours, the seamless capsule of Comparative Example the dye was eluted dye was seamless capsule of Example elution rate of about 2 times, then opened the gap continued. Comparative Example 1 were free of nuclear seamless capsule soy lecithin Comparative Example 2 shows the blending amount of soy lecithin was 2.01 parts by weight of the low amount of experimentation. The blending amount of soybean lecithin in Example 1 was 3.35 parts by weight, from figure 2 Point of view, we can see there is a big gap ...
Embodiment 3
[0079] (A) a liquid nucleus: JC stirring 47.17 parts by weight of oil at 60 ℃, 11.79 parts by weight of edible fat (IOI Oleo manufactured Witocan42 / 44: mp 43 ± 3 ℃) and 3.35 parts by weight of soybean lecithin were dissolved in a uniform, mixing 4.69 parts by weight loxoprofen sodium hydrate (CAS80382-23-6) the resulting lysate, the resulting suspension to a solution as a core.
[0080] (B) a liquid for intermediate layer: mixing 4.65 parts by weight of oil-JC, parts of the Witocan42 / 44 and 0.7 parts by weight of soybean lecithin 4.65 wt., Liquid for the intermediate layer.
[0081] (c) Older solution: mix 17.48 parts by weight of gelatin at 60 ° C [Jellystregth, gel strength): 280 Brony (Bloom)], 4.60 parts by weight of glycerol, 0.92 parts by weight of low methoxy Base (LM) pectin and 85.20 parts by weight of pure water, as the outermost liquid.
[0082] Was added dropwise from an inner nozzle of concentric triple nozzle with the core liquid, and the nozzle from a solution o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com