Method for co-producing D-tagatose, arabitol and galactitol and application
A technology of arabinitol and galactitol, applied in the field of bioengineering, can solve the problems of high biochemical oxygen demand of whey powder, not being well utilized, waste of resources and the environment, and achieve high-value utilization and reduce Process cost, the effect of increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
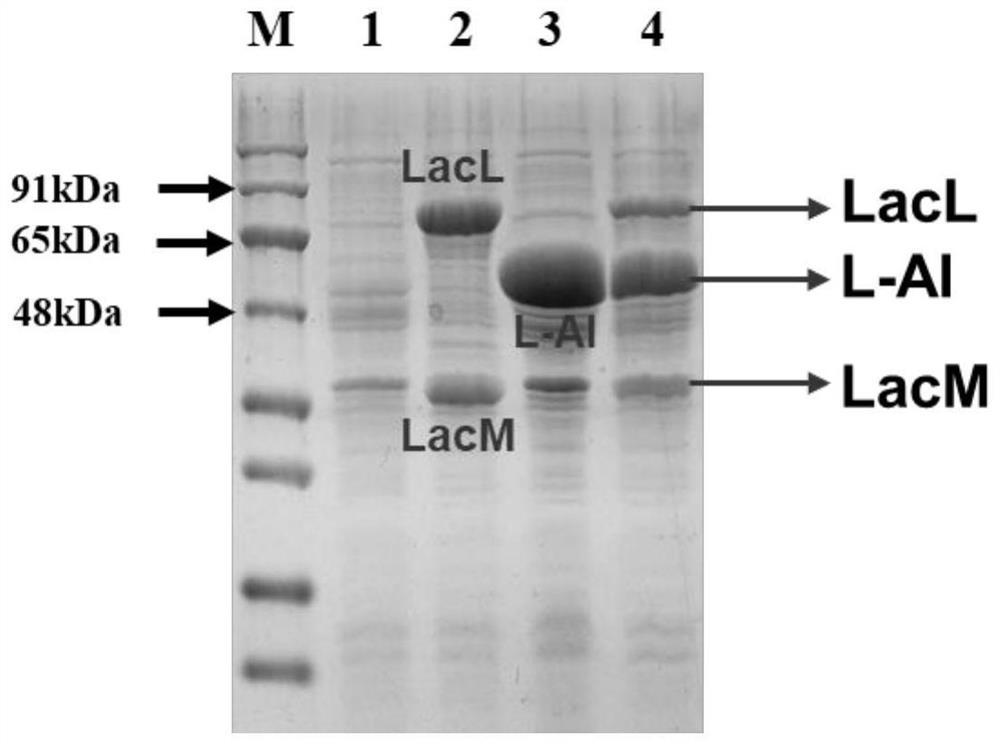
[0036] Embodiment 1. Construction and dual-enzyme expression of overexpressed recombinant β-gal and L-AI genetically engineered bacteria:
[0037] (1) Acquisition of araA gene:
[0038] According to the araA gene (GenBankNo.CP055123.1) sequence of Lactobacillus plantarum encoding L-AI published in Genbank and the characteristics of the homology arm of the vector pANY1, the upstream primer 5'-taactttaagaaggagatatacatATGTTATCAGTACCTGATTATGAG-3' (SEQ ID NO.1) and the downstream primer 5'-aggagctcccatggaggTTACTTTAAGAATGCCTTAGTCAT-3' (SEQ ID NO.2), the first half of which is the upstream and downstream homology arms homologous to the pANY1 plasmid.
[0039] (2) Using Lactobacillus plantarum CY.6 (Lactobacillus plantarum CY.6, screening method references Zhang G, Zabed H M, J Yun, et al. Bioresource Technology, 2020, 305:123010.) genomic DNA as a template, using step (1) The target gene araA was amplified by PCR using the upstream primers and downstream primers synthesized in , and...
Embodiment 2
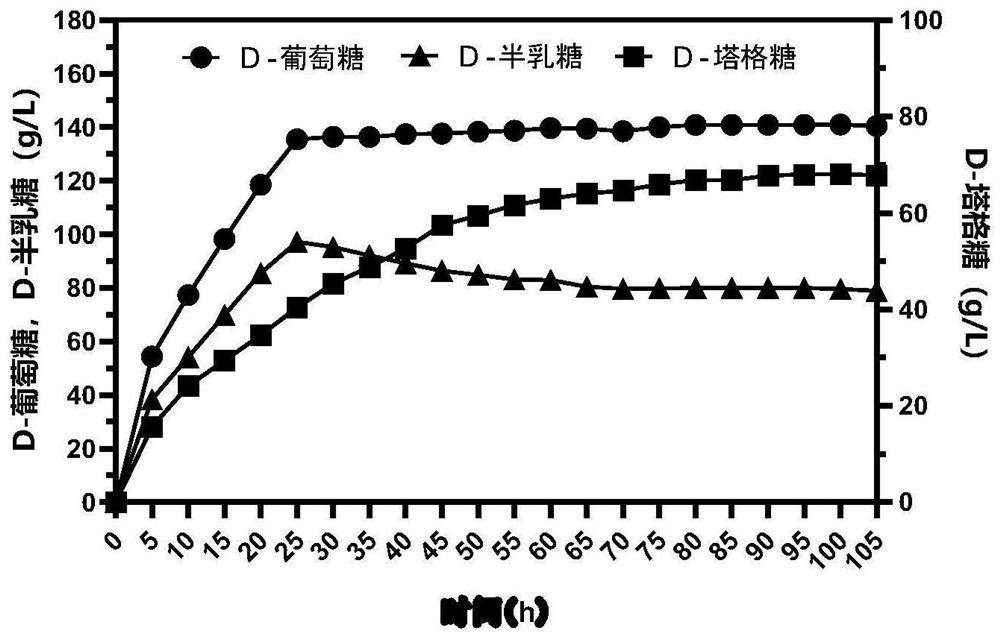
[0049] Embodiment 2. whey powder fed-batch production D-tagatose:
[0050] (1) Inoculate the genetically engineered bacteria E.coli BL21 / β-gal-L-AI in Kan-Cl-LB, shake and culture at 37°C and 200rpm until the logarithmic phase of growth, and prepare a seed solution; then the seeds When inoculated in Kan-Cl-LB with an inoculum volume fraction of 1% and cultivated to an OD of 0.6-0.8, the inducer IPTG was added to make the final concentration 1mM, and then overnight at 25°C and 120rpm Induced joint expression of β-gal and L-AI.
[0051] (2) The bacterial solution successfully expressing the dual enzymes was refrigerated and centrifuged at 8000rpm at 4°C for 10 minutes to collect the bacterial cells, and 100mM sodium phosphate buffer solution (pH 7.0) with 2% Triton-x-100 (by volume fraction) was used to incubate at room temperature After treatment for 30 min, the bacteria were collected by centrifugation, and the bacteria were washed twice with pH 7.0, 100 mM sodium phosphate b...
Embodiment 3
[0054] Example 3: Fermentation of D-Glucose and D-Galactose by Maggi Mekki Yeast to Co-produce D-Arabitol and Galactitol
[0055] (1) Streak the preserved Metschnikowia pulcherrima E1 (CCTCC NO:M2019256) on YPD solid medium (glucose 20g / L, peptone 20g / L, yeast powder 10g / L, agar 15g / L) After 48 hours of static culture in an incubator at 30°C, a single colony was picked and inoculated in YPD liquid medium, and cultured with shaking at 30°C and 200 rpm for 16 hours to the logarithmic phase to prepare a seed solution.
[0056] (2) Use a refrigerated centrifuge at 8000rpm and 4°C for 10 minutes to remove the cells in the D-tagatose conversion solution produced by the pre-transformation, take the supernatant and heat to boil to denature the residual protein in the solution, and then centrifuge again and take the supernatant as The substrate solution of yeast fermentation reaction in this step, and the pH of the substrate solution is adjusted to about 6.5.
[0057] (3) Prepare fermen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com