Preparation method of Fe2B-Co2B composite material-based sodium borohydride hydrolysis hydrogen production catalyst
A composite material and sodium borohydride technology are applied in the field of preparation of catalysts based on Fe2B-Co2B composite sodium borohydride hydrolysis for hydrogen production, which can solve the problems affecting wide application and high cost of precious metals, and achieve excellent stability and high sodium borohydride water. Analyzing the effect of hydrogen performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
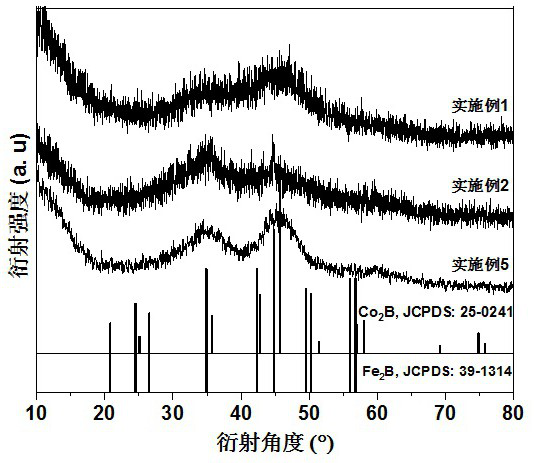
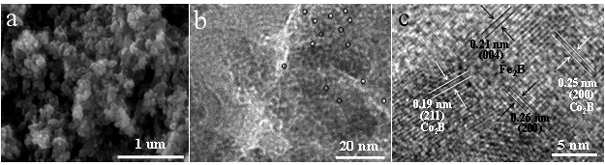
[0021] 1. Preparation of Co 2 B catalyst:
[0022] Weigh 2 mmol of cobalt chloride hexahydrate, 60 mmol of sodium chloride and 60 mmol of urea and mix them thoroughly in a mortar, then put the ground samples in a blast oven at 60 °C for 4 hours. The dried sample was thoroughly ground again, and 12 mmol of sodium borohydride solid was added at room temperature, and the reaction was mixed for 1 hour. Subsequently, the reaction product was fully washed with deionized water, and the obtained product was collected and placed in a vacuum oven at 60 °C for 12 hours to obtain the product Co 2 B catalyst.
[0023] 2. Catalyst test:
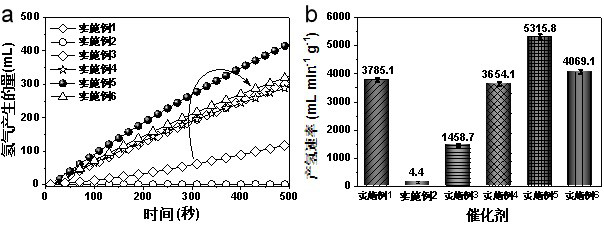
[0024] Add 50 mL of 150 mM NaBH to a 100 mL three-neck round bottom flask 4 aqueous solution (containing 0.4 wt % NaOH), and then put the three-necked round-bottom flask in a water bath at 25 °C and keep stirring for 30 minutes until the reading of the electronic balance connected to the test does not change. Keep the above conditions of constant temp...
Embodiment 2
[0029] 1. Preparation of Fe 2 B catalyst:
[0030] Weigh 2 mmol of ferric chloride hexahydrate, 60 mmol of sodium chloride and 60 mmol of urea and mix them thoroughly in a mortar and grind them evenly, then place the ground samples in a blast oven at 60 °C for 4 hours. The dried sample was thoroughly ground again, and 12 mmol of sodium borohydride solid was added at room temperature, and the reaction was mixed for 1 hour. Subsequently, the reaction product was fully washed with deionized water, and the obtained product was collected and placed in a vacuum oven at 60 °C for 12 hours to obtain the product Fe 2 B catalyst.
[0031] 3. Catalyst test:
[0032] Add 50 mL of 150 mM NaBH to a 100 mL three-neck round bottom flask 4 aqueous solution (containing 0.4 wt% NaOH), then put the three-necked round-bottom flask in a water bath at 25 °C and keep stirring for 30 minutes until the reading of the electronic balance connected to the test does not change. Keep the above conditio...
Embodiment 3
[0034] 1. Preparation of Co 2 B‒Fe 2 B catalyst (Co:Fe=1:1):
[0035] Weigh 1 mmol of cobalt chloride hexahydrate, 1 mmol of ferric chloride hexahydrate, 60 mmol of sodium chloride and 60 mmol of urea and mix them thoroughly in a mortar, then place the ground sample in a 60 °C Insulate in forced air oven for 4 hours. The dried sample was thoroughly ground again, and 12 mmol of sodium borohydride solid was added at room temperature, and the reaction was mixed for 1 hour. Subsequently, the reaction product was fully washed with deionized water, and the obtained product was collected and placed in a vacuum oven at 60 °C for 12 hours to obtain the product Co 2 B‒Fe 2 B catalyst.
[0036] 2. Catalyst test:
[0037] Add 50 mL of 150 mM NaBH to a 100 mL three-neck round bottom flask 4 aqueous solution (containing 0.4 wt% NaOH), then put the three-necked round-bottom flask in a 25 °C water bath and keep stirring for 30 minutes until the reading of the electronic balance connect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com