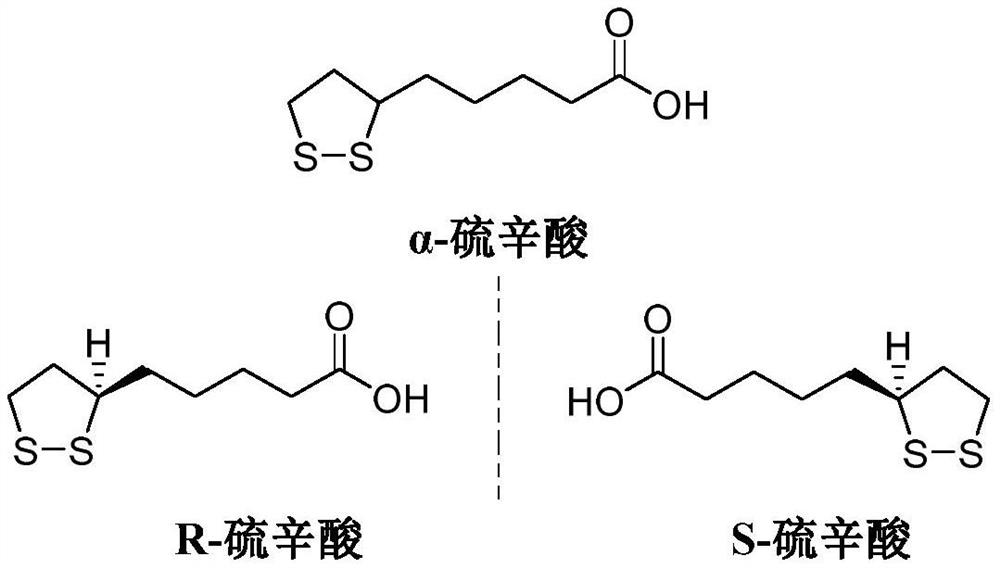
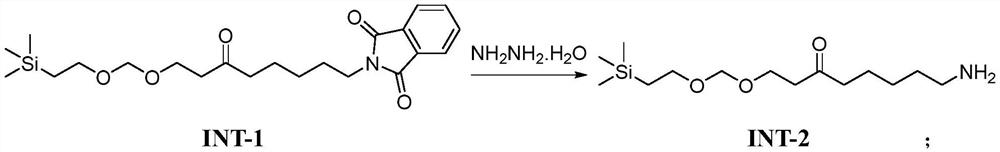
Synthesis method of R-lipoic acid
A synthetic method, technology of lipoic acid, applied in the direction of organic chemical methods, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of equipment corrosion, complicated process, expensive production cost, etc., and achieve The effect of meeting the use requirements, reasonable technical solutions, and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
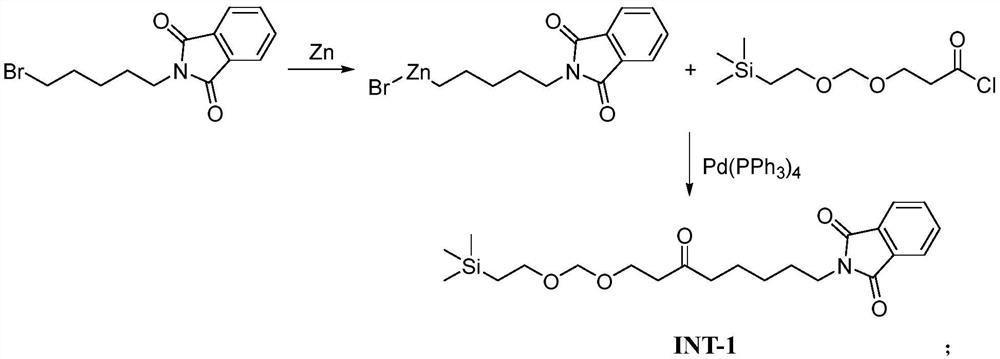
[0043] A) Preparation of Intermediate-1:
[0044] In a 5L reaction flask, add anhydrous tetrahydrofuran (100mL), under nitrogen protection and stirring at room temperature, add anhydrous lithium chloride (14.0g, 0.33mol), zinc powder (44.0g, 0.67mol), stir for 10min, add A mixed solvent of 1,2-dibromoethane (10mL) and anhydrous tetrahydrofuran (100mL), was added trimethylchlorosilane (1.2g, 11mmol), heated to 40°C and stirred for 30min, cooled to room temperature, added N-( 5-Bromopentyl)phthalimide (65.0g, 0.22mol), the temperature of the reaction mixture was raised to 40°C for 3h, then cooled to normal temperature, the insoluble matter was removed by suction filtration, the filtrate was collected, and tetrakis(triphenylphosphine)palladium ( 12.7g, 11.0mol), stirred at room temperature for 30min, added 3-{[2-(trimethylsilyl)ethoxy]methoxy}propionyl chloride (52.4g, 0.22mol) in anhydrous tetrahydrofuran solution (100mL) , keep warm at 20°C for 6 hours, after the reaction is c...
Embodiment 2
[0076] A) Preparation of Intermediate-1:
[0077] In a 5L reaction flask, add anhydrous tetrahydrofuran (60mL), under nitrogen protection and stirring at room temperature, add anhydrous lithium chloride (16.0g, 0.38mol), zinc powder (55.0g, 0.84mol), stir for 10min, add A mixed solvent of 1,2-dibromoethane (5mL) and anhydrous tetrahydrofuran (50mL), was added trimethylchlorosilane (1.5g, 14mmol), heated to 50°C and stirred for 30min, cooled to room temperature, added N-( 5-Bromopentyl)phthalimide (55.0g, 0.19mol), the temperature of the reaction mixture was raised to 50°C for 2h, then cooled to normal temperature, the insoluble matter was removed by suction filtration, the filtrate was collected, and tetrakis(triphenylphosphine)palladium ( 16.0g, 14mmol), stirred at room temperature for 30min, added 3-{[2-(trimethylsilyl)ethoxy]methoxy}propionyl chloride (44.3g, 0.19mol) in anhydrous tetrahydrofuran solution (50mL), Insulate at 30°C and react for 4 hours. After the reaction i...
Embodiment 3
[0098] A) Preparation of Intermediate-1:
[0099]In a 5L reaction flask, add anhydrous tetrahydrofuran (100mL), under nitrogen protection and stirring at room temperature, add anhydrous lithium chloride (37.0g, 0.87mol), zinc powder (138.0g, 2.11mol), stir for 10min, add A mixed solvent of 1,2-dibromoethane (10mL) and anhydrous tetrahydrofuran (100mL), was added trimethylchlorosilane (3.5g, 32mmol), heated to 60°C and stirred for 30min, cooled to room temperature, added N-( 5-Bromopentyl)phthalimide (105.0g, 0.35mol), the temperature of the reaction mixture was raised to 60°C for 1h, then cooled to room temperature, the insoluble matter was removed by suction filtration, the filtrate was collected, and tetrakis(triphenylphosphine)palladium ( 40.0g, 35mmol), stirred at room temperature for 30min, added 3-{[2-(trimethylsilyl)ethoxy]methoxy}propionyl chloride (84.7g, 0.35mol) in anhydrous tetrahydrofuran solution (100mL), Keep warm at 35°C and react for 2 hours. After the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com