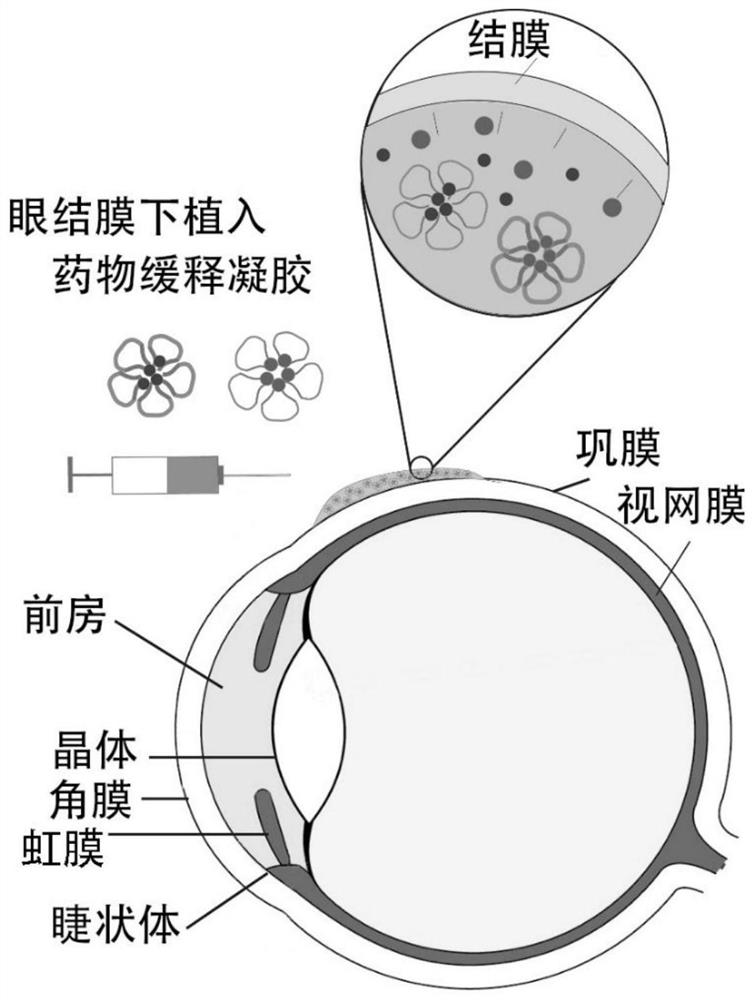
Drug sustained-release gel for subconjunctival implantation and preparation method of drug sustained-release gel
A slow-release gel and drug technology, which is applied to the drug slow-release gel implanted under the conjunctiva and the field of preparation thereof, can solve the problems of tissue trauma, unfavorable antibacterial and anti-inflammatory treatment, tissue toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example provides a drug slow-release gel for subconjunctival implantation and a preparation method thereof.
[0041] The drug sustained-release gel includes levofloxacin, prednisolone and an injectable composite gel carrier. Wherein, the injectable composite gel carrier includes polylactic acid-glycolic acid copolymer-polyethylene glycol-polylactic acid-glycolic acid triblock copolymer nanomicelle; the levofloxacin and prednisolone are uniformly dispersed in In the nanomicelle. Table 1 lists the drug sustained-release gel drug and dispersion of the present embodiment. As shown in Table 1, according to the mass percentage, the proportion of prednisolone is 0.25% (w / w), the proportion of levofloxacin is 0.15% (w / w), and the concentration of triblock copolymer is 25% (w / w). ).
[0042] The preparation method of the drug slow-release gel for subconjunctival implantation provided by this embodiment comprises the following steps:
[0043] Disperse levofloxacin (1.5 m...
Embodiment 2
[0046] This example provides a drug slow-release gel for subconjunctival implantation and a preparation method thereof.
[0047] The drug sustained-release gel includes levofloxacin, prednisolone and an injectable composite gel carrier. Wherein, the injectable composite gel carrier includes polylactic acid-glycolic acid copolymer-polyethylene glycol-polylactic acid-glycolic acid triblock copolymer nanomicelles and metal hydroxide nanoparticles, and the levofloxacin and Prednisolone is uniformly dispersed in the nanoparticles. Table 1 lists the drug sustained-release gel drug and dispersion of the present embodiment. As shown in Table 1, according to the mass percentage, the proportion of prednisolone is 0.15% (w / w), and the proportion of levofloxacin is 0.15% (w / w), which is used to load the metal hydroxide of prednisolone and levofloxacin The concentration of nanoparticle was 2.0% (w / w), the concentration of copolymer was 22% (w / w), and the proportion of composite gel as ca...
Embodiment 3
[0053] This example provides a drug slow-release gel for subconjunctival implantation and a preparation method thereof.
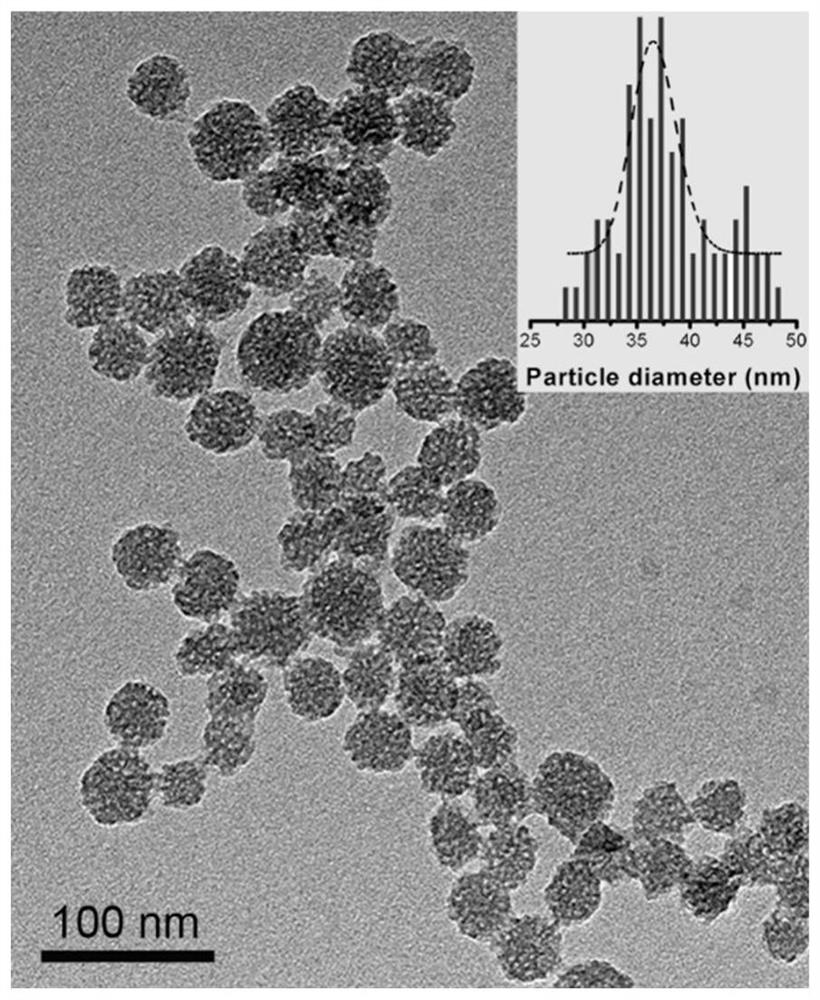
[0054] The drug sustained-release gel includes levofloxacin, prednisolone and an injectable composite gel carrier. This embodiment focuses on the slow delivery of anti-inflammatory and antibacterial drugs at the same time, so the anti-inflammatory drug prednisolone and the antibacterial drug levofloxacin are simultaneously loaded in porous silica nanoparticles, and then the drug-loaded nanoparticles are dispersed in polylactic acid-hydroxyl Acetic acid copolymer-polyethylene glycol-polylactic acid-glycolic acid triblock copolymer nanomicelles. Porous silica nanoparticles such as image 3 shown.
[0055] Table 1 lists the drug sustained-release gel drug and dispersion of the present embodiment. As shown in Table 1, according to the mass percentage, the proportion of levofloxacin is 0.1% (w / w), and the proportion of prednisolone is 0.15% (w / w). The concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com