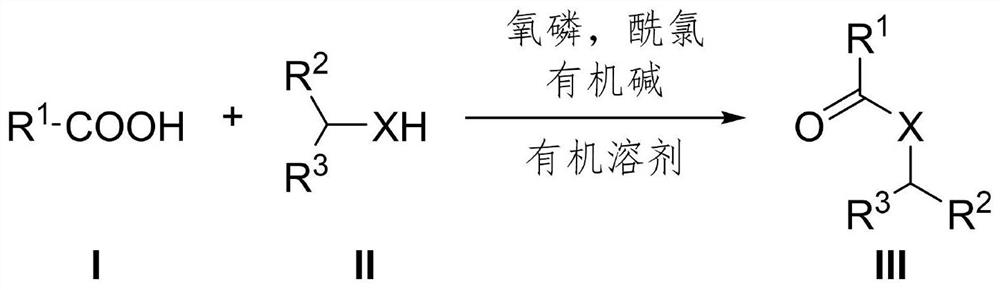
Preparation method of amide or ester compound
A technology for ester compounds and amine compounds, applied in the field of preparation of amides or ester compounds, can solve the problems of difficult removal, expensive condensing agents or catalysts, small racemization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071]
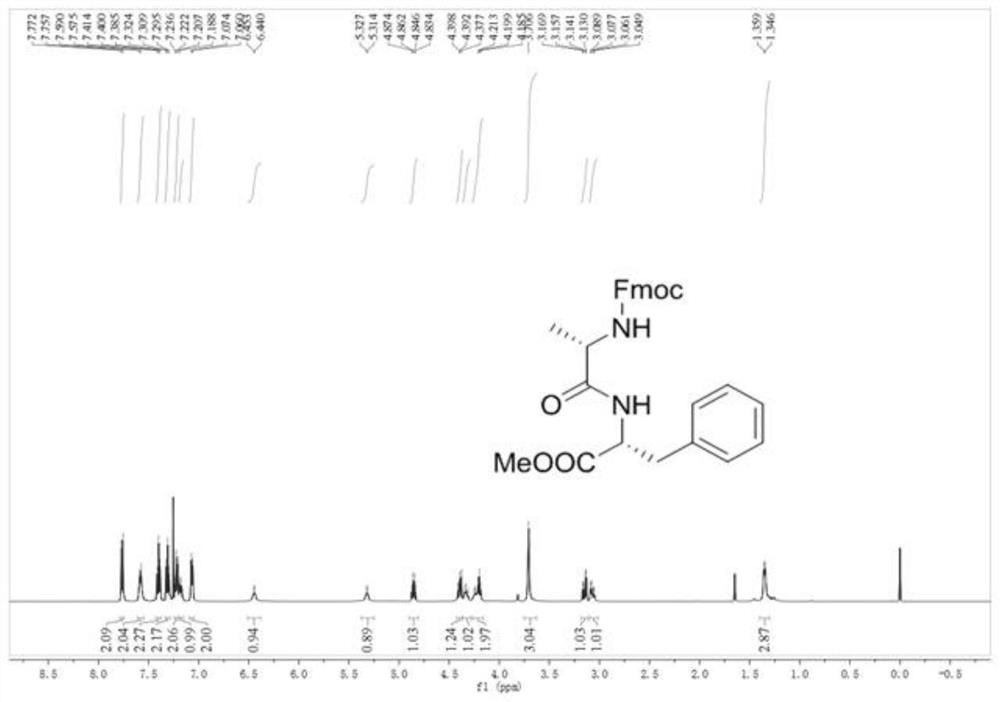
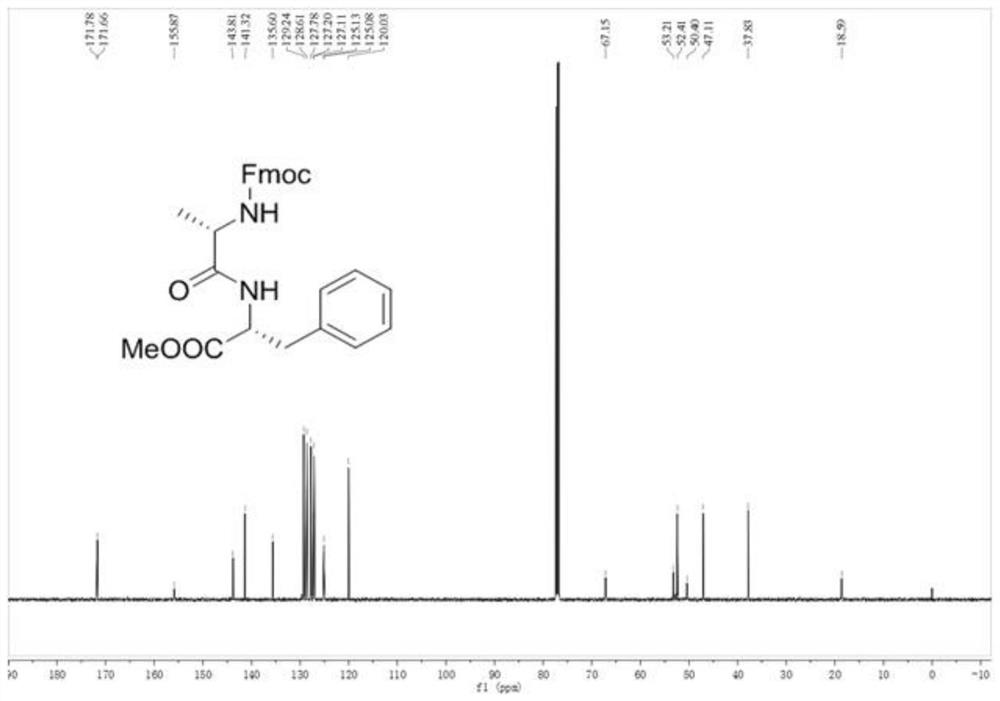
[0072] In a 25mL round bottom flask, dissolve triphenylphosphine oxide (0.056g, 0.2mmol) in 1.0mL of 1,2-dichloroethane, add thionyl chloride (0.109 mL, 1.5mmol), N-Fmoc-L-alanine (0.311g, 1.0mmol), L-phenylalanine methyl ester (0.269g, 1.5mmol) and triethylamine (0.277mL, 2.0 mmol), The stirring reaction was continued for 10 min at 25°C.
[0073] After the reaction was over, 50 mL of ethyl acetate was added to the reaction solution, and 30 mL of saturated Na 2 CO 3 The solution was washed 2 times and 20mL saturated brine was washed 2 times, and the organic phase was collected after separation, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, and evaporation of the solvent under reduced pressure gave the crude product; the crude product was purified by column chromatography, and the mixture of ethyl acetate (EA) and petroleum ether (PE) with a volume ratio of 30:70 was used as an eluent for elution, The eluate was collected and dist...
Embodiment 2-4
[0079] All the other are identical with embodiment 1, difference is: the molar mass of triphenylphosphine oxide in embodiment 2,3,4 is respectively 0.1mmol, 0.5mmol and 1.0mmol, the content of condensing agent triphenylphosphine oxide The corresponding relationship with the experimental results is shown in Table 1 below.
[0080] Table 1 Correspondence between the content of the condensing agent triphenylphosphine oxide and the experimental results
[0081]
[0082] Analysis of experimental results: as can be seen from the results of Examples 1-4 and Table 1, the content of the condensing agent triphenylphosphine oxide has a significant impact on the reaction system, and the inventors have found that within a certain range, with the The increase of the condensing agent triphenylphosphine oxide content, the productive rate of product increases thereupon, tends to be stable subsequently; Therefore comprehensively consider the productive rate of the cost of raw material and pr...
Embodiment 5
[0084]
[0085] In a 25mL round bottom flask, dissolve triphenylphosphine oxide (0.056g, 0.2mmol) in 1.0mL 1,2-dichloroethane, and add oxalyl chloride (0.127mL, 1.5 mmol), N-Fmoc-L-leucine (0.353g, 1.0mmol), L-leucine tert-butyl ester (0.281g, 1.5mmol) and triethylamine (0.277mL, 2.0mmol), at 25°C The stirring reaction was continued for 10 min.
[0086] After the reaction was over, 50 mL of ethyl acetate was added to the reaction solution, and 30 mL of saturated Na 2 CO 3 The solution was washed 3 times and 20mL saturated brine twice, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent under reduced pressure to obtain a crude product. The crude product was purified by column chromatography, using a mixture of ethyl acetate (EA) and petroleum ether (PE) with a volume ratio of 30:70 as the eluent for elution, and the eluate was collected and distilled under reduced pressure again to obtain a white solid 0.460g, the dipeptide p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com