Aripiprazole pharmaceutical co-crystal and preparation method thereof
An aripiprazole and drug technology, applied in the field of aripiprazole drug co-crystal and preparation thereof, can solve the problems of adverse reactions, poor solubility of aripiprazole, etc., and achieve the advantages of reducing solubility and muscle irritation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation method of aripiprazole drug co-crystal, the method is a grinding method, which specifically includes the following steps: putting aripiprazole and resveratrol into a mortar for grinding in equimolar amounts, and adding a ketone or alkyl nitrile solvent before grinding , manual grinding for 30min, in Vacuum-dried to obtain the drug co-crystal of aripiprazole.
[0020] The mass ratio of aripiprazole to resveratrol is 1:1. The co-crystal of aripiprazole and resveratrol is used in the preparation of various antipsychotic drugs, and the schizophrenia includes positive symptoms, negative symptoms, cognitive impairment and bipolar disorder.
[0021] The instrument that detects drug eutectic structure and performance among the present invention is as follows:
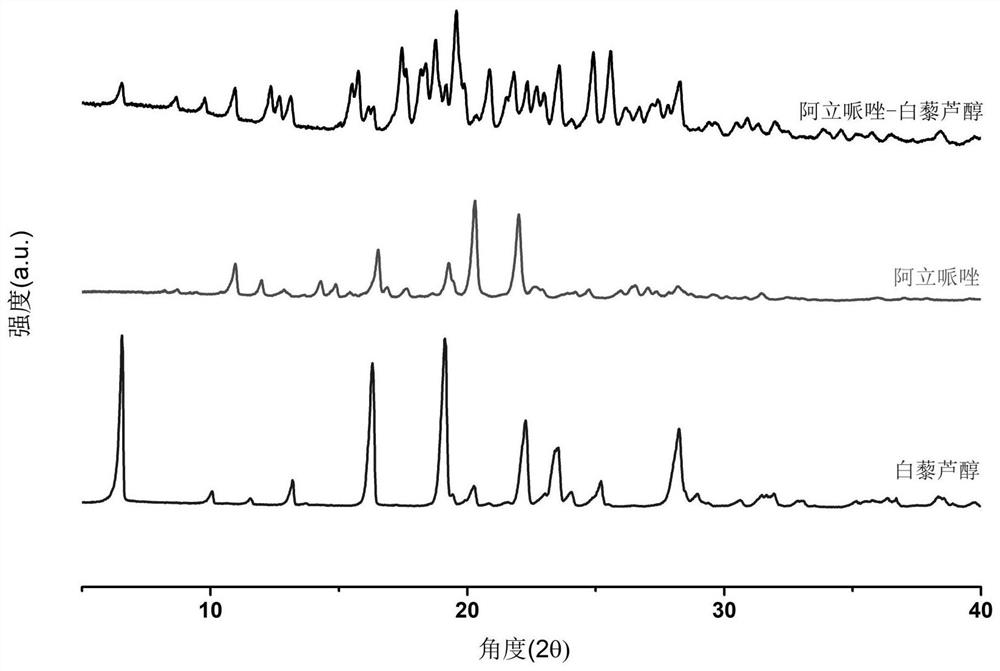
[0022] 1. X-ray powder diffractometer, produced by PANalytical Company in the Netherlands, model X’Pert PRO MPD, Cu-K(α), tube voltage 40kV, tube current 40mA, scanning speed 2° / min;
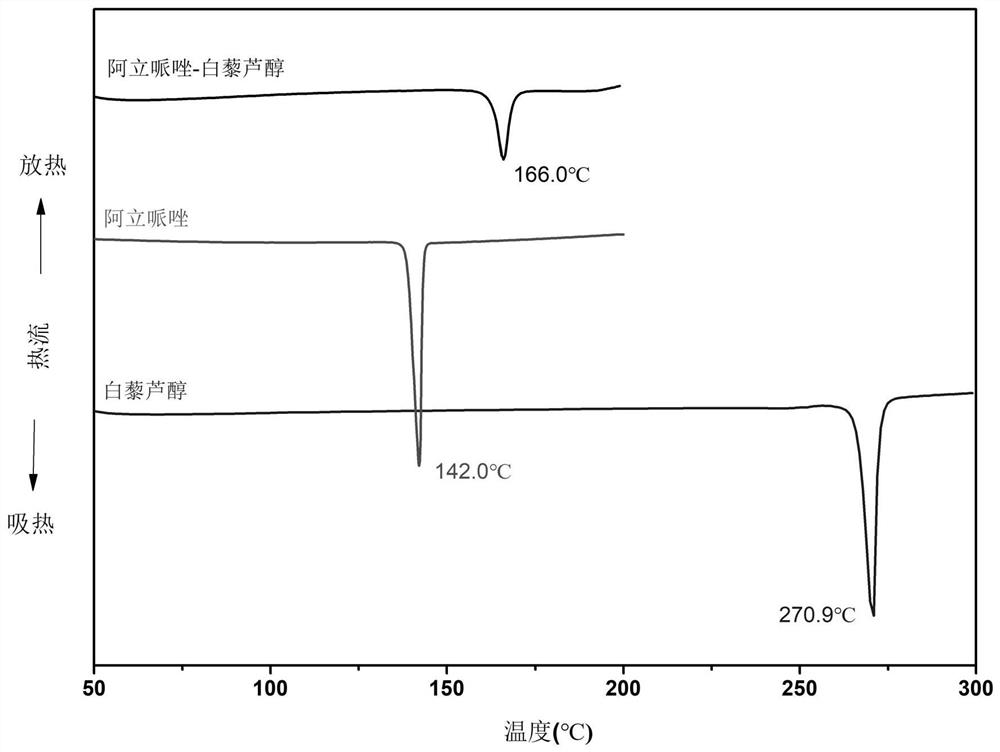
[0023] 2. Diffe...
Embodiment 1
[0027] Accurately weigh 224 mg (0.5 mmol) of aripiprazole and 114 mg (0.5 mmol) of resveratrol, place them in a mortar, add acetonitrile dropwise and grind for 30 min to obtain a white powder, dry in vacuum at 50°C for 4 h, and collect the solid, namely It is the co-crystal of aripiprazole and resveratrol (ARI-RSV).
Embodiment 2
[0029] Accurately weigh 224mg (0.5mmol) of aripiprazole and 114mg (0.5mmol) of resveratrol, put them in a mortar, add methyl isobutyl ketone dropwise and grind for 30min to obtain a white powder, and dry in vacuum at 50°C for 4h , to collect the solid, which is the co-crystal of aripiprazole and resveratrol (ARI-RSV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com