High-cerium-content scandium-doped gadolinium iron garnet magneto-optical crystal as well as preparation method and application thereof
A magneto-optical crystal and garnet technology, which is applied in the field of high-cerium-containing scandium-doped gadolinium-iron garnet magneto-optic crystal and its preparation, can solve the problems of easy corrosion of solvents, harmfulness to human body and environment, etc., and achieve excellent magneto-optical performance, Good crystallinity and good optical uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
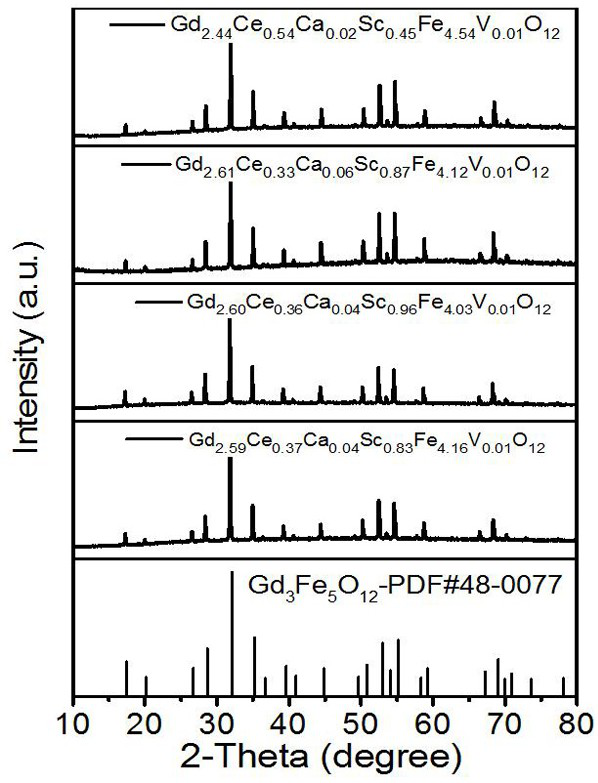
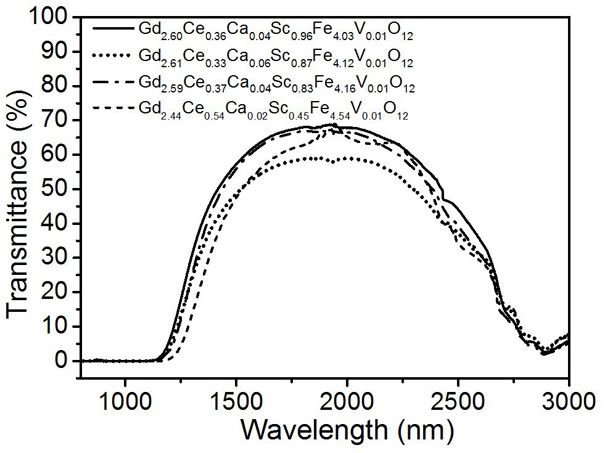
[0027] Magneto-optic crystal Gd with high cerium content and scandium-doped gadolinium iron garnet 2.60 Ce 0.36 Ca 0.04 sc 0.96 Fe 4.03 V 0.01 o 12 And preparation method thereof, concrete steps are as follows:
[0028] (1) Synthesis of polycrystalline raw materials: Weigh high-purity Gd according to the stoichiometric ratio 2 o 3 , Fe 2 o 3 Drugs, after being ground and mixed evenly, pressed into tablets, pre-sintered at 800°C for 10 hours, then sintered at 1300°C for 10 hours, after taking out, ground and pressed tablets, and then sintered at 1300°C for 10 hours to obtain Gd 3 Fe 5 o 12 polycrystalline material. The stoichiometric ratio of CeO 2 , NH 4 VO 3 The powder was mixed evenly, pressed into tablets, and then heated and sintered. During sintering, the temperature was kept at 200°C, 400°C, 670°C and 800°C for 2 hours respectively to obtain CeVO 4 polycrystalline powder. The stoichiometric CaCO 3 , Fe 2 o 3 The powder is pre-sintered at 900°C for 10h...
Embodiment 2
[0033] Magneto-optic crystal Gd with high cerium content and scandium-doped gadolinium iron garnet 2.61 Ce 0.33 Ca 0.06 sc 0.87 Fe 4.12 V 0.01 o 12 And preparation method thereof, concrete steps are as follows:
[0034] (1) Synthesis of polycrystalline raw materials: Weigh high-purity Gd according to the stoichiometric ratio 2 o 3 , Fe 2 o 3 Drugs, after being ground and mixed evenly, pressed into tablets, pre-sintered at 800°C for 10 hours, then sintered at 1300°C for 10 hours, after taking out, ground and pressed tablets, and then sintered at 1300°C for 10 hours to obtain Gd 3 Fe 5 o 12 polycrystalline material. The stoichiometric ratio of CeO 2 , NH 4 VO 3 The powder was mixed evenly, pressed into tablets, and then heated and sintered. During sintering, the temperature was kept at 200°C, 400°C, 670°C and 800°C for 2 hours respectively to obtain CeVO 4 polycrystalline powder. The stoichiometric CaCO 3 , Fe 2 o 3The powder is pre-sintered at 900°C for 10h ...
Embodiment 3
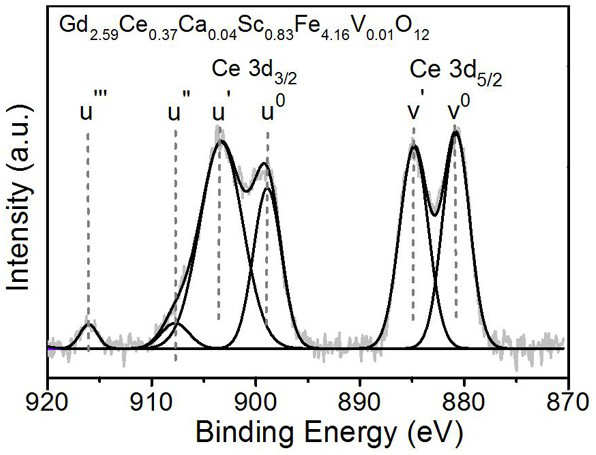
[0039] Magneto-optic crystal Gd with high cerium content and scandium-doped gadolinium iron garnet 2.59 Ce 0.37 Ca 0.04 sc 0.83 Fe 4.16 V 0.01 o 12 And preparation method thereof, concrete steps are as follows:
[0040] (1) Synthesis of polycrystalline raw materials: Weigh high-purity Gd according to the stoichiometric ratio 2 o 3 , Fe 2 o 3 Drugs, after being ground and mixed evenly, pressed into tablets, pre-sintered at 800°C for 10 hours, then sintered at 1300°C for 10 hours, after taking out, ground and pressed tablets, and then sintered at 1300°C for 10 hours to obtain Gd 3 Fe 5 o 12 polycrystalline material. The stoichiometric ratio of CeO 2 , NH 4 VO 3 The powder was mixed evenly, pressed into tablets, and then heated and sintered. During sintering, the temperature was kept at 200°C, 400°C, 670°C and 800°C for 2 hours respectively to obtain CeVO 4 polycrystalline powder. The stoichiometric CaCO 3 , Fe 2 o 3 The powder is pre-sintered at 900°C for 10h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com