Preparation method of photosensitive biphenyl diamine monomer
A biphenylenediamine and photosensitivity technology, which is applied in the field of preparation of photosensitive biphenylenediamine monomers, can solve the problems of limited types of graft polymers, great difficulty in industrialization, reduced product yield and the like, and achieves a simple synthesis method. Safe, continuous preparation, the effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 A kind of preparation method of photosensitive biphenyl diamine monomer
[0032] The synthetic route of photosensitive biphenyl diamine monomer is as follows:
[0033]
[0034] The preparation method is carried out in the following order of steps:
[0035] (1): Synthesis of compound A (2,2'-dinitro-4,4'-dihydroxybiphenyl)
[0036] When the 4,4'-dihydroxyl group is directly nitrated, due to the electron-donating effect of the phenolic hydroxyl group, it will directly introduce two nitro groups at the 3,3' position, and if you want to introduce two nitro groups at the 2,2' or 2,3' position The nitro group needs to change the electron-donating effect of the position of its phenolic hydroxyl group, so p-nitrobenzenesulfonyl chloride is used to react with the phenolic hydroxyl group to generate a strong electron-withdrawing p-nitrobenzenesulfonyl group, and the strong electron-withdrawing property of the newly generated group It can make the 2,2' position i...
Embodiment 2
[0060] Embodiment 2 A kind of preparation method of photosensitive biphenyl diamine monomer
[0061] The synthetic route of photosensitive biphenyl diamine monomer is as follows:
[0062]
[0063] The preparation method is carried out in the following order of steps:
[0064] (1): Synthesis of compound A (2,3'-dinitro-4,4'-dihydroxybiphenyl)
[0065] a1. Add 9.3g of 4,4'-dihydroxybiphenyl and 5g of sodium bicarbonate into a single-necked bottle filled with 200ml of ethanol in an ice-water bath, and add 6g of p-nitrobenzenesulfonyl chloride at a rate of 1 drop / second under magnetic stirring Slowly added dropwise to the reaction system;
[0066] a2. At 0°C, stir magnetically and react for 12 hours, then filter the ethanol and wash the product with 200 ml of deionized water three times to obtain 18.1 g of the crude intermediate product A-1;
[0067] a3. Slowly add 70ml of dilute nitric acid with a mass fraction of 68% into the one-necked bottle, place it in an ice-water bat...
Embodiment 3
[0088] Embodiment 3 A kind of preparation method of photosensitive biphenyl diamine monomer
[0089] The synthetic route of photosensitive biphenyl diamine monomer is as follows:
[0090]
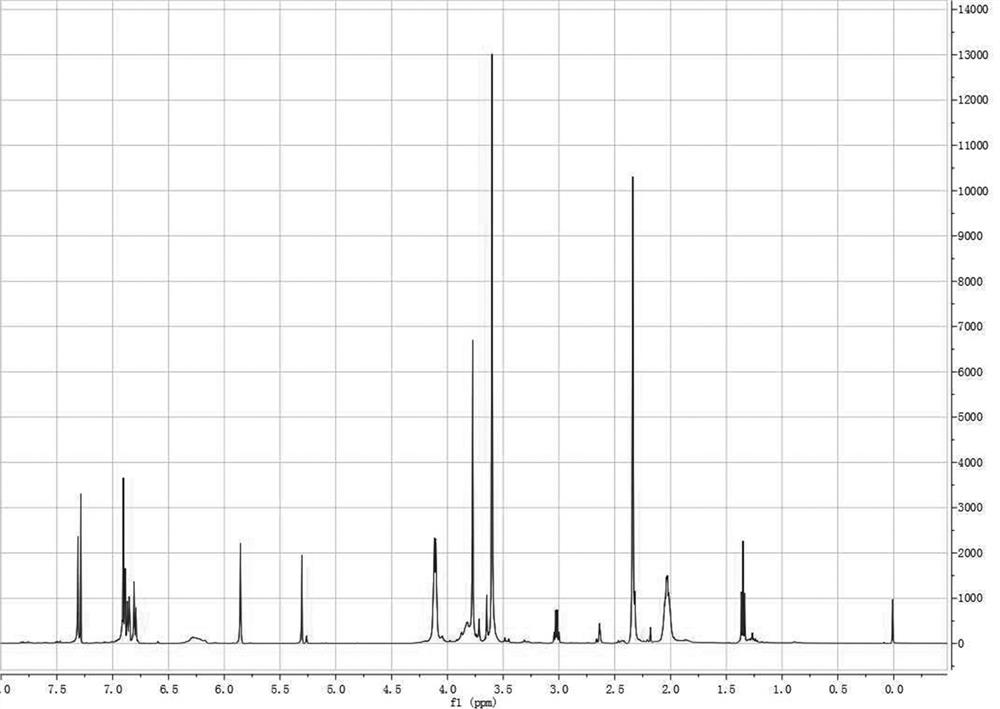
[0091] according to figure 1 It can be seen that the structure of the photosensitive biphenylenediamine monomer prepared by this method is consistent with the structure of the final product of the design route through the comparison of the chemical shift of the specific characteristic proton and the corresponding integral value, which strongly proves that The practicability of the photosensitive biphenyl diamine monomer synthesis method proposed by the present invention;
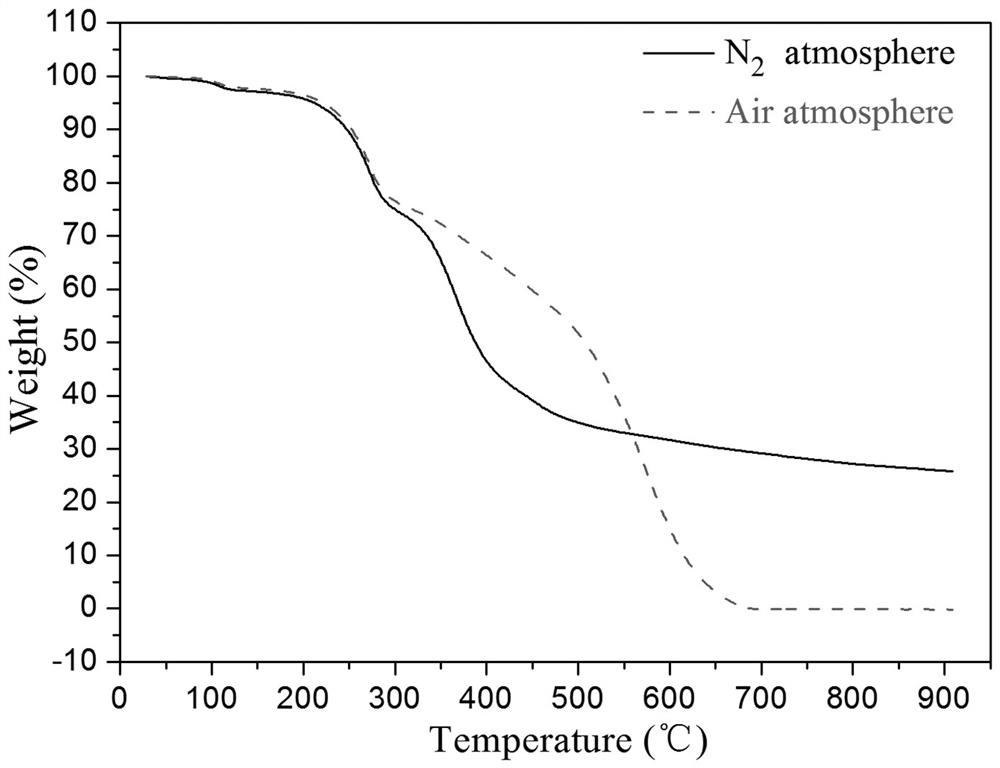
[0092] according to figure 2 As shown, the prepared photosensitive biphenyl diamine monomer has high thermal stability no matter in the nitrogen atmosphere or in the air atmosphere. When the ambient temperature reaches 210 ° C, the thermal stability of the photosensitive diamine monomer The weight loss is only abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com