Pharmaceutical composition, and preparation method and use thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as non-compliance with drug administration, aggravating patients' nervousness, anxiety and fear, and delaying diagnosis and treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
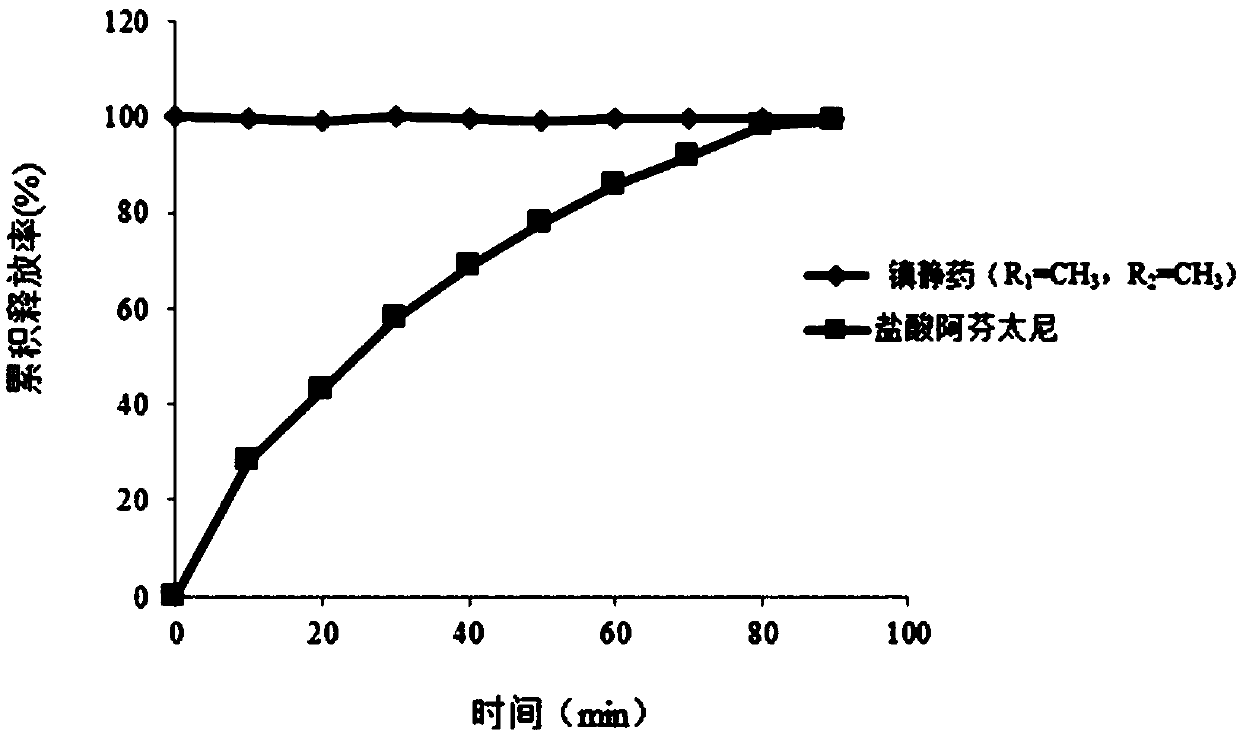
[0098] Embodiment 1 preparation contains sedative (formula (I), R 1 =CH 3 , R 2 =CH 3 , besylate) and alfentanil hydrochloride nasal microsphere formulation
[0099] 1. take by weighing 2g chitosan, be dissolved in the acetic acid aqueous solution of 5% in 100ml volume ratio, obtain the chitosan solution of 2% (g / 100ml); Take by weighing 50mg alfentanil hydrochloride and be dissolved in 10ml 2% ( g / 100ml) chitosan solution, adjust the pH to 2.9~3.7 with 1mol / L NaOH, as the water phase;
[0100] ② Take 100ml of liquid paraffin containing 2% (v / v) Span80 as the oil phase; when the stirring speed of electric stirring is 1200r / min, and the temperature is room temperature, slowly add the water phase to the oil phase through a syringe, Emulsify for 15 minutes to make a W / O emulsion;
[0101] ③At room temperature, with a stirring speed of 400r / min, slowly add 1ml of cross-linking agent glutaraldehyde, solidify for 90min, and let it stand until there are no bubbles; min and an o...
Embodiment 2
[0104] Example 2 Preparation of nasal microsphere preparations containing dexmedetomidine hydrochloride and dexketamine hydrochloride
[0105] ① Take 2g chitosan and dissolve it in 100ml volume ratio of 5% acetic acid aqueous solution to obtain 2% chitosan solution; weigh 46mg dexketamine hydrochloride and dissolve it in 10ml 2% (g / 100ml) chitosan Sugar solution, adjust the pH to 2.9-3.7 with 1mol / L HCl, as the water phase;
[0106] ② Take 100ml of liquid paraffin containing 2% (v / v) Span80 as the oil phase; when the stirring speed of electric stirring is 1200r / min, and the temperature is room temperature, slowly add the water phase to the oil phase through a syringe, Emulsify for 15 minutes to make a W / O emulsion;
[0107] ③At room temperature, with a stirring speed of 400r / min, slowly add 1ml of cross-linking agent glutaraldehyde, solidify for 90min, and let it stand until there are no bubbles; min and an outlet temperature of 75°C for spray drying to obtain dexketamine hy...
Embodiment 3
[0110] Embodiment 3 preparation contains sedative (formula (I), R 1 =CH 3 , R 2 =CH 3 , tosylate) and fentanyl citrate nasal microsphere formulation
[0111] 1. Take by weighing 2g chitosan, be dissolved in 100ml and be 5% (v / v) in the acetic acid aqueous solution, obtain the chitosan solution of 2% (g / 100ml); Take by weighing 56mg fentanyl citrate and dissolve in 10ml of 2% (g / 100ml) chitosan solution, adjusted to pH 2.8-3.5 with 1mol / L HCl, as the water phase;
[0112] ② Take 100ml of liquid paraffin containing 2% (v / v) Span80 as the oil phase; when the stirring speed of electric stirring is 1200r / min, and the temperature is room temperature, slowly add the water phase to the oil phase through a syringe, Emulsify for 15 minutes to make a W / O emulsion;
[0113] ③At room temperature, with a stirring speed of 400r / min, slowly add 1ml of cross-linking agent glutaraldehyde, solidify for 90min, and let it stand until there are no bubbles; min and outlet temperature of 75°C t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com