Targeting element and preparation method and application thereof
A targeting and component technology, applied in the field of medicine, can solve the problems of no active targeting, poor absorption of target organs in the body for targeted drug delivery, and reduced utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Material
[0110]
[0111]
[0112] The material of lipid is cholesterol, egg yolk lecithin, soybean lecithin, cephalin, sphingomyelin, PC (phosphatidylcholine), EPG (egg phosphatidylglycerol), SPG (soybean phosphatidylglycerol), Distearoylphosphatidylethanolamine (DSPE), dipalmitoylphosphatidylethanolamine (DPPE), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylcholine (DOPC), distearoylphosphatidylcholine Alkaline (DSPC), Dimyristoylphosphatidylcholine (DMPC), Dilinoleoylphosphatidylcholine (DLPC), Dioleoylphosphatidylglycerol (DOPG), Dipalmitoylphosphatidylglycerol (DPPG), Dimely Myristoylphosphatidylcholine (DMPC), Dilauroylphosphatidylglycerol (DLPG), Cetyltrimethylammonium Bromide (CTAB), Dimethyl Dioctadecylammonium Bromide (DDAB), One or more of 1,2-dioleoyl-3-trimethylammoniumpropane (chloride salt) (DOTAP).
[0113] In this embodiment, the amino acid of the spacer material is one of arginine, asparagine, aspartic acid, glutamic aci...
Embodiment 2
[0114] Example 2 Preparation of Targeting Elements
[0115] The targeting material and spacer material in Example 1 were synthesized, and the synthesis of mannose and PEGn was taken as an example here.
[0116] Take diethylene glycol, p-toluenesulfonyl chloride and triethylamine, dissolve in DCM, react at room temperature for 24 hours, and separate the product TosOC by column chromatography 2 h 4 OC 2 h 4 OH (compound 1). Take compound 1 and pentaacetylated mannose and its derivatives and boron trifluoride ether (BF 3 ·Et 2 O) dissolved in DCM, reacted at room temperature for 24h, separated by column chromatography to obtain the product Aco-M-OC 2 h 4 OC 2 h 4 OTos (compound 2), M is mannose and its derivatives.
Embodiment 3
[0117] Example 3 Preparation of Targeting Vector
[0118] The targeting element in Example 2 was further synthesized with the basic nano-preparation material, and the synthesis of mannose, PEGn and cholesterol was taken as an example here.
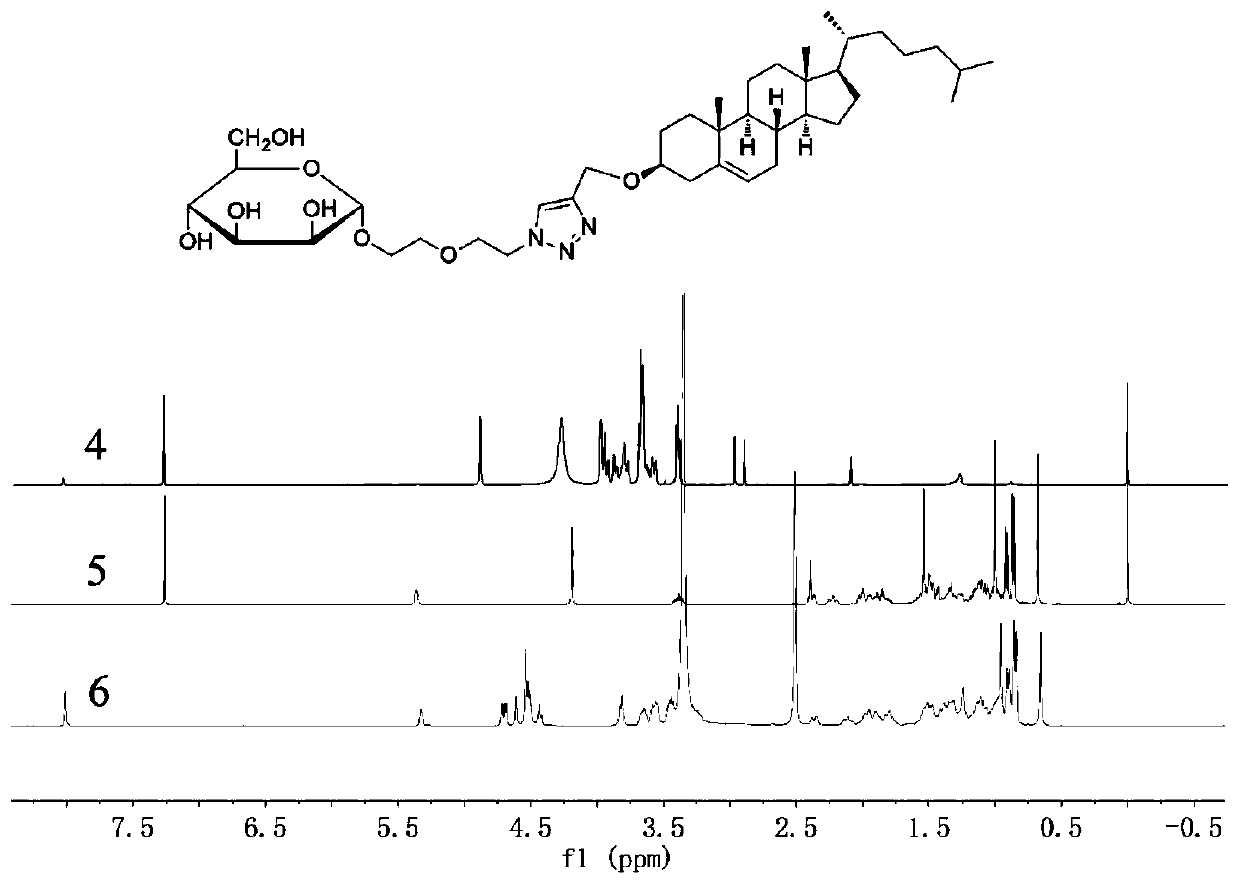
[0119] Take the compound prepared in Example 2 and sodium azide, dissolve in DMF, react at 60°C for 24h, and separate by silica gel column chromatography to obtain the solid product Aco-M-OC 2 h 4 OC 2 h 4 N 3 (Compound 3). Dissolve compound 3 in a methanol solution of sodium methoxide, react at room temperature for 3 h, and concentrate to obtain the product M-OC 2 h 4 OC 2 h 4 N 3 (compound 4). Cholesterol, propyne bromide and sodium hydrogen were dissolved in a mixed solution of ether and DMF, reacted at room temperature for 24 hours, and separated by silica gel column chromatography to obtain the solid product Chol-CH 2 CCH (Compound 5). Dissolve compound 4, compound 5 and cuprous iodide in DMF, react at room temperature for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com