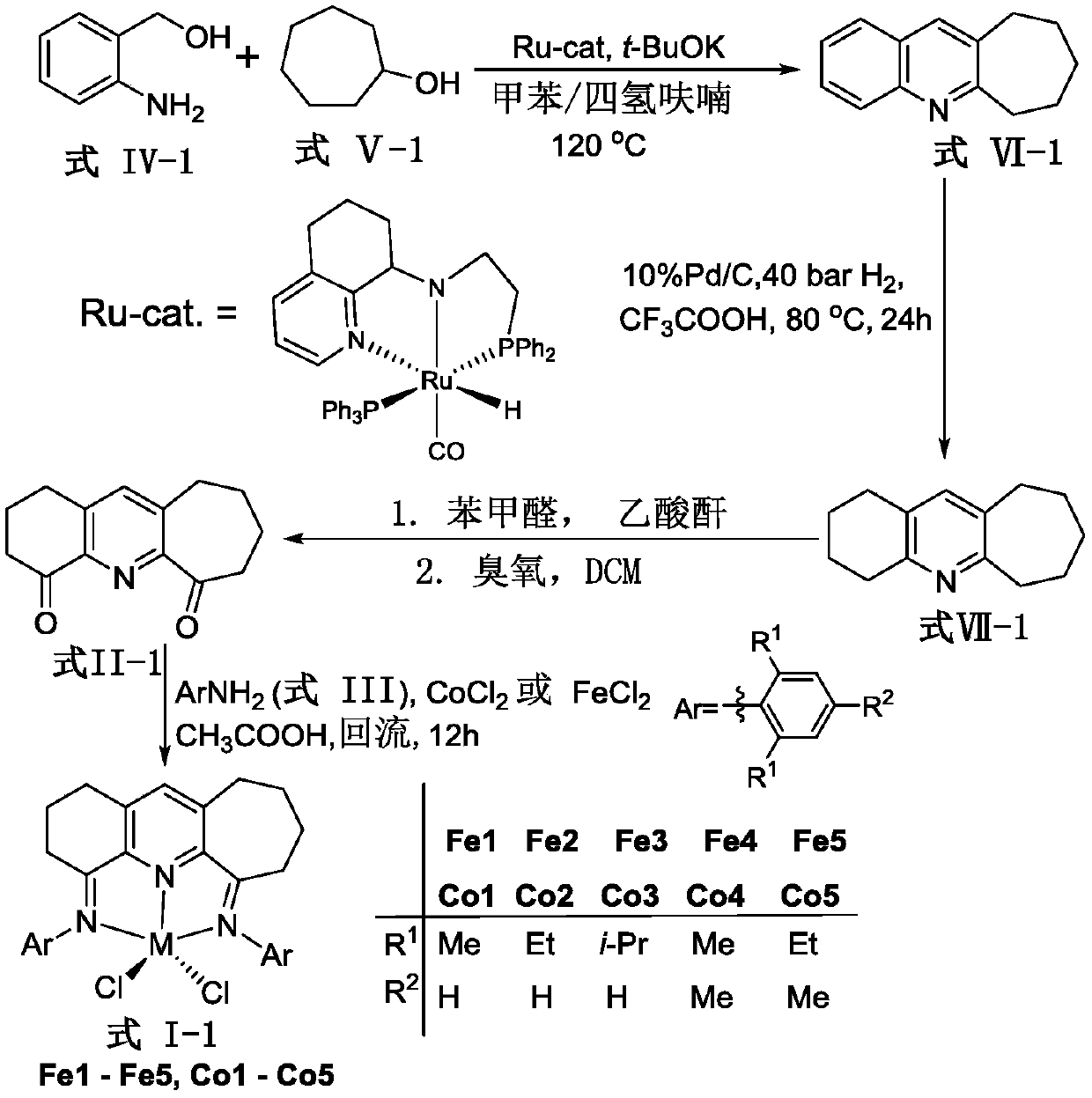
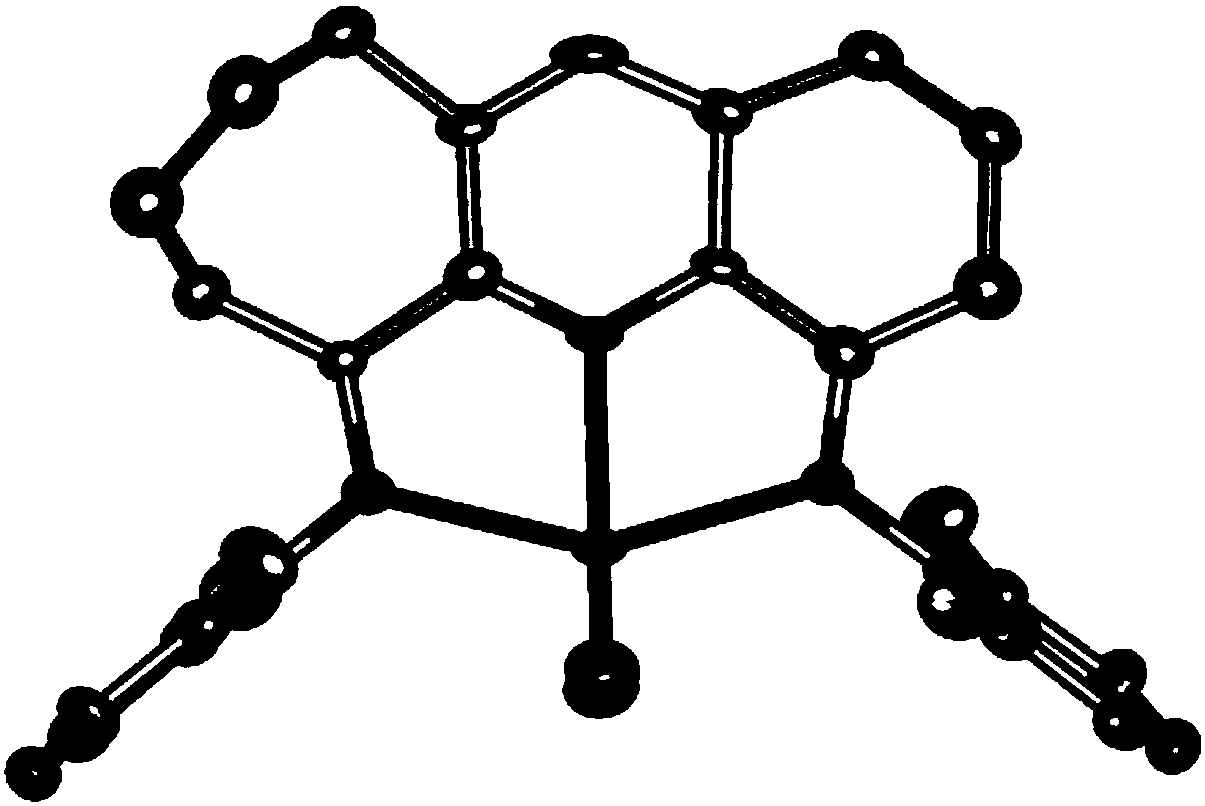
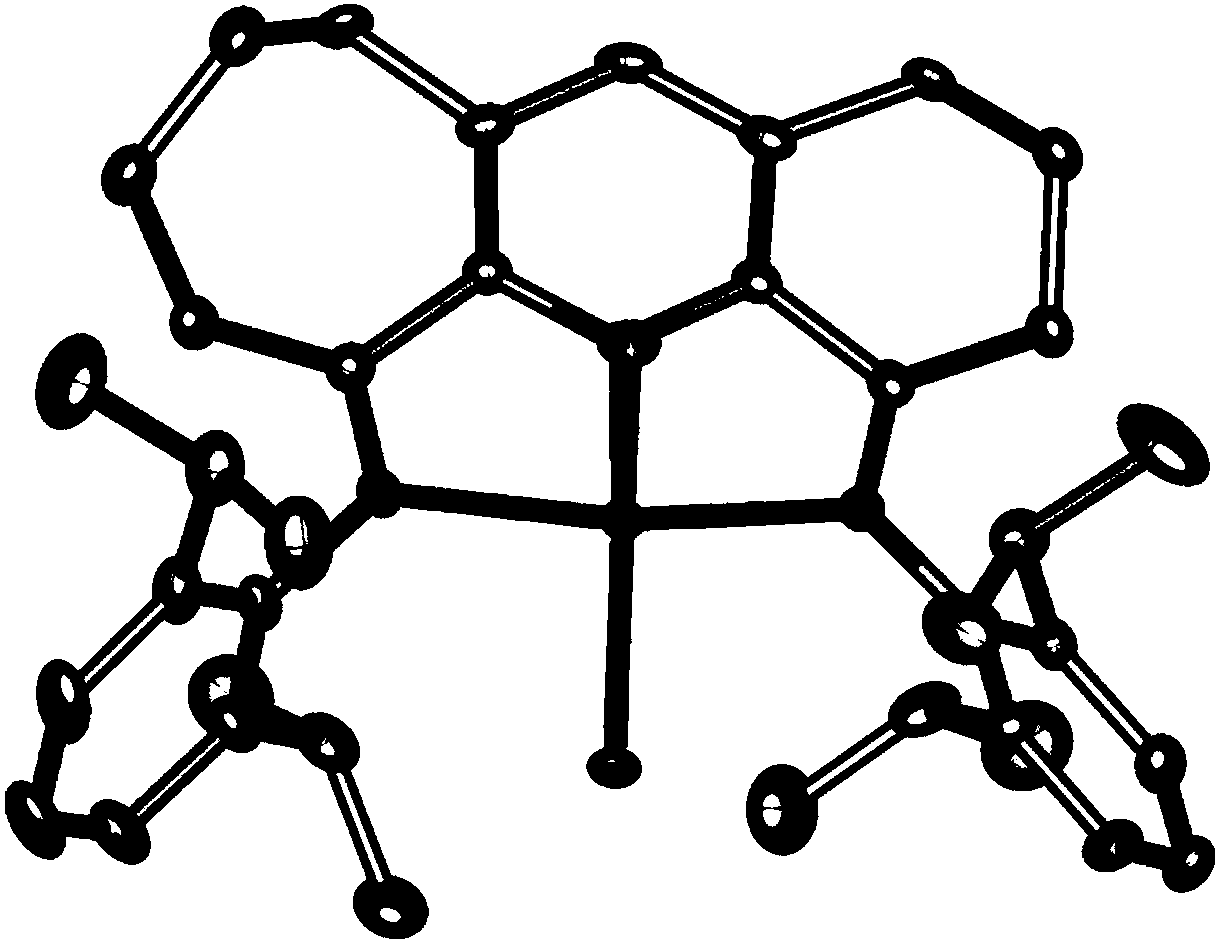
Pyridylimine group complex containing asymmetric fused rings and preparation method and application of pyridylimine group complex
A kind of compound and cycloalkyl technology, applied in the field of asymmetric condensed ring pyridineimine complex and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1 Preparation of Formula VI-1 Intermediate 7,8,9,10-tetrahydro-6H-cycloheptaquinoline compound
[0115]
[0116] With 123.0g (1.0mol) o-aminobenzyl alcohol represented by IV-1 formula, 136.8g (1.20mol) V-1 cycloheptanol and 134.4g (1.20mol) potassium tert-butoxide and 380mg (0.5mmol) Ru-cat .Dissolved in a mixed solution of 150mL tetrahydrofuran and 750mL toluene, under nitrogen atmosphere, at 120°C, stirred and refluxed for 72h. TLC detects that the reaction is complete. Cool to room temperature, remove tetrahydrofuran and toluene under reduced pressure to obtain a brown oil, add 200mL of water to it, stir for 0.5h, then extract with ethyl acetate (2×400mL), combine the organic phases, wash with deionized water (2×100mL ) and washed with anhydrous Na 2 SO 4 After drying and filtering, the solvent was removed from the filtrate to obtain 180g of red oil, which was dissolved in dichloromethane (100mL), and then a large amount of n-hexane (300mL) was added, a...
Embodiment 2
[0122] Example 2 Preparation of Formula VII-1 Intermediate 2,3,4,6,7,8,9,10-octahydro-1H-cycloheptaquinoline compound
[0123]
[0124] 145.0g (0.73mol) formula VI-1, 14.5g (10%) Pd / C and 500mL trifluoroacetic acid are put into 1000mL stainless steel alloy autoclave, replace with hydrogen three times, under 40bar H 2 Under pressure, at 80°C, the reaction was stirred for 24h. Stop the reaction, release excess hydrogen, filter the reaction solution, distill the filtrate to remove trifluoroacetic acid to obtain a yellow oil, add 10% NaOH aqueous solution, adjust pH≈14, extract with dichloromethane (2×100mL), and use anhydrous Na 2 SO 4 Drying, filtering to obtain a light yellow liquid, and distilling off the solvent to obtain 118.0 g of 2,3,4,6,7,8,9,10-octahydro-1H-cycloheptaquinoline compound shown in formula VII-1. The yield is 80%, melting point: 45–46°C.
[0125] The structural confirmation data are as follows:
[0126] 1 H NMR (500MHz, CDCl 3 )δ7.08(s,1H),3.01–2.9...
Embodiment 3
[0130] Example 3 Preparation of intermediate 2,3,7,8,9,10 hexahydro-1H-cycloheptaquinoline-4,6-dione compound represented by formula II-1
[0131]
[0132] 2,3,4,6,7,8,9,10-octahydro-1H-cycloheptaquinoline compound represented by 60.0g (0.30mol) VII-1 formula was dissolved in 318.0g (3.00mol) benzene Formaldehyde and 240.0 g (2.40 mol) of acetic anhydride were reacted under reflux at 180° C. for 72 h under a nitrogen atmosphere. After the reaction was detected by TLC, it was cooled to room temperature, and a vacuum distillation device was set up, and the reaction solution was subjected to vacuum distillation (100° C., 10 mm Hg) to remove unreacted benzaldehyde, acetic anhydride, and by-products. After vacuum distillation, the brown oil was dissolved in 1200 mL of dichloromethane. The reaction solution was cooled to below -40°C and passed through dry O 3 / O 2 , carry out ozonation reaction until the solution turns light yellow or light blue, react for about 3 hours, TLC d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com