Preparation method of prism copper oxide-zinc oxide catalyst
A zinc oxide, prismatic technology, which is applied in the field of preparation of prismatic copper oxide-zinc oxide catalysts, can solve the problem of no specific shape, and achieve the effects of good thermal stability, regular product shape, and low activation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
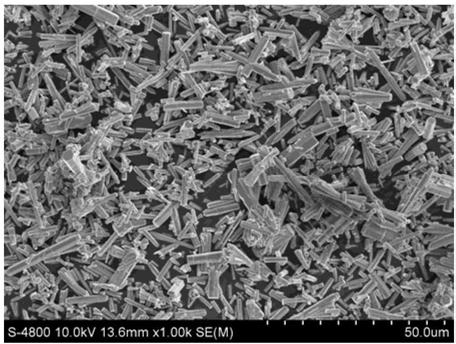
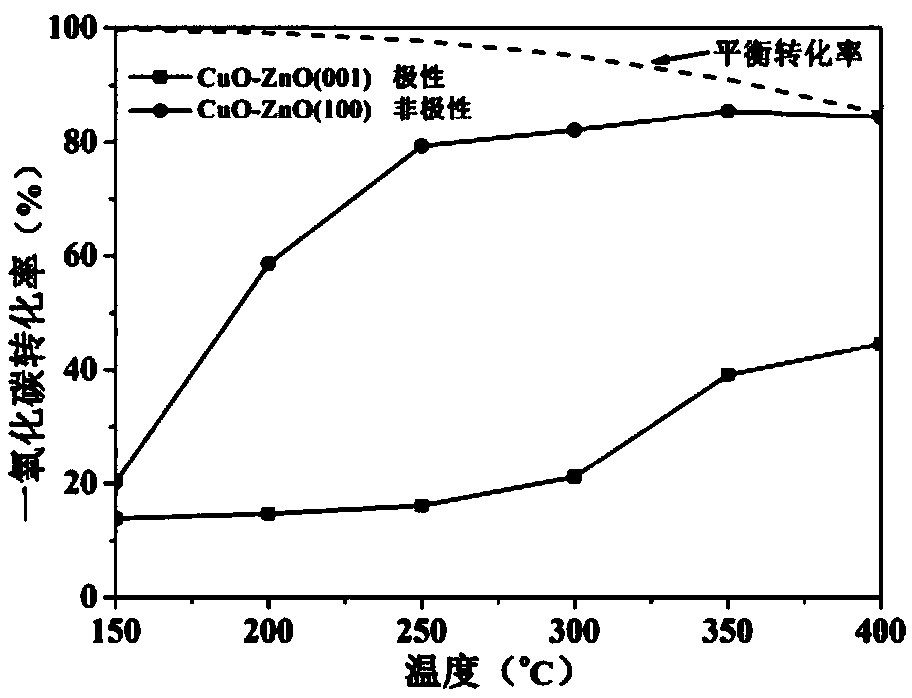
[0026] Mainly exposing the polar {001} crystal plane zinc oxide carrier, the preparation method of the prismatic copper oxide-zinc oxide catalyst of the present invention comprises the following steps:
[0027] 1) Dissolve 3g of zinc acetate dihydrate in 12ml of absolute ethanol while stirring to obtain mixed solution A, and simultaneously dissolve 1.9g of hexamethylenetetramine in deionized water to obtain mixed solution B, then mix The mixed solution A is added to the mixed solution B, and after stirring, 0.5 g of polyvinylpyrrolidone K30 is added, and the mixture is uniformly stirred to obtain a mixed solution C. The volumes of absolute ethanol and deionized water are the same;
[0028] 2) After ultrasonic treatment for 0.5h, the mixed solution C was transferred to a reaction kettle with a polytetrafluoroethylene liner for heating reaction, wherein the temperature of the heating reaction was 50°C to 150°C, the heating reaction time was 12h, and then cooled After reaching ro...
Embodiment 2
[0031] Mainly expose the preparation of non-polar {100} crystal plane zinc oxide carrier, the preparation method of prismatic copper oxide-zinc oxide catalyst according to the present invention comprises the following steps:
[0032] 1) Dissolve 3g of zinc acetate dihydrate in 120ml of absolute ethanol while stirring to obtain mixed solution A, and simultaneously dissolve 1.9g of hexamethylenetetramine in deionized water to obtain mixed solution B, then mix The mixed solution A is added to the mixed solution B, and after stirring, 5 g of polyvinylpyrrolidone K30 is added, and the mixture is uniformly stirred to obtain a mixed solution C. The volume of absolute ethanol and deionized water is the same;
[0033] 2) After ultrasonic treatment for 0.5h, the mixed solution C was transferred to a reaction kettle with a polytetrafluoroethylene liner for heating reaction, wherein the temperature of the heating reaction was 90°C, and the heating reaction time was 12h, and then cooled to ...
Embodiment 3
[0038] The preparation method of the prismatic copper oxide-zinc oxide catalyst of the present invention comprises the following steps:
[0039] 1) Dissolve 2.0g of zinc acetate dihydrate in 5ml of absolute ethanol while stirring to obtain mixed solution A, and simultaneously dissolve 1.0g of hexamethylenetetramine in deionized water to obtain mixed solution B, and then Add mixed solution A to mixed solution B, add 0.1 g of polyvinylpyrrolidone K30 after stirring, and stir evenly to obtain mixed solution C, the volumes of absolute ethanol and deionized water are the same;
[0040] 2) After ultrasonic treatment for 0.5h, the mixed solution C was transferred to a reaction kettle with a polytetrafluoroethylene liner for heating reaction, wherein the temperature of the heating reaction was 50°C, the heating reaction time was 6h, and then cooled to room temperature. Carry out centrifugal separation, and then alternately wash with deionized water and anhydrous ethanol until the wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com