Chicken infectious anemia virus VP1 and VP2 expression genetic recombination fowlpox virus living-vector vaccine
A chicken infectious anemia, live vector vaccine technology, applied in the direction of virus/phage, vaccine, virus, etc., can solve the problems of high cost, low virus titer, rare use of inactivated vaccine, etc., to reduce economic losses, Avoid the effect of horizontal transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
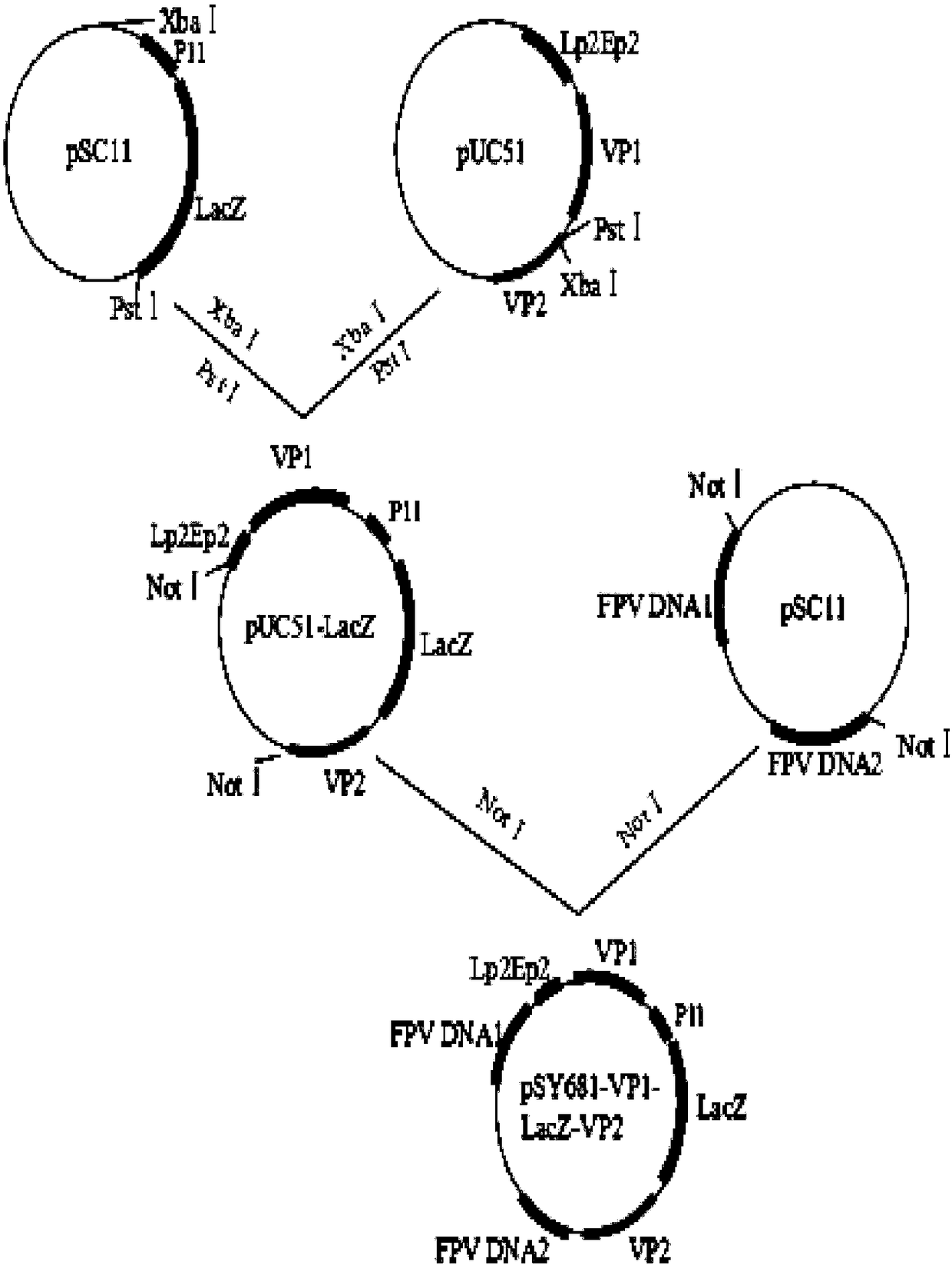
[0030] Example 1 Chicken Infectious Anemia Virus VP1, VP2 Gene Recombination Fowlpox Virus Live Vector Vaccine rFPV-VP1-VP2 Construction
[0031] a. Isolation and identification of CAV epidemic strains
[0032] The nucleotide sequences of 36 chicken infectious anemia virus reference strains isolated from around the world were downloaded from the NCBI database, and compared with the whole genome sequences of 12 chicken infectious anemia virus strains recently isolated in Guangdong by our laboratory. Using MegAlign software, the homology comparative analysis was carried out on the complete gene sequences, VP1 gene nucleotide sequences and deduced VP1 amino acid sequences of 12 isolates and 36 reference viruses. The results of evolutionary analysis of the whole gene sequence showed that the phylogenetic tree was composed of three branches: branch II included Chinese isolates DQ141673 and AY999018; branch III included Chinese isolates DQ124936 and KU221054, American isolate AF3119...
Embodiment 2
[0049] Example 2: Titer and Growth Curve Determination of Recombinant Fowlpox Virus
[0050] a. Titer of recombinant fowlpox virus
[0051] Take 100 μL and dilute to 10 -3 、10 -4 、10 -5 、10 -6 、10 -7 、10 -8 The virus solution of the recombinant fowlpox virus obtained in Example 1 was inoculated into the treated CEF cells, and each dilution was repeated 4 times, and placed in 37 ° C, 5% CO 2 cultured in an incubator. When the CEF cells have obvious lesions, record the number of plaques that appear, calculate the average value of four replicate plaques for each dilution, and convert the virus plaque forming units (PFU) in the virus solution according to the number of plaques. The results showed that rFPV-VP1-VP2 and FPV titers were greater than 10 6 PFU / mL (see Table 1).
[0052] Table 1 Virus amplification titer
[0053]
[0054] b. Determination of Growth Curve of Recombinant Fowlpox Virus
[0055] Infect cells with recombinant fowlpox virus and fowlpox virus res...
Embodiment 3
[0070] Example 3: Detection of immune efficacy of recombinant fowlpox virus co-expressing chicken infectious anemia virus VP1 and VP2 genes
[0071] a. ELISA detects the change of CAV antibody titer in breeder chicken serum
[0072] 50 90-day-old SPF breeder chickens were inoculated with FPV and rFPV-VP1-VP2 wings, and the inoculation dose was 10 5 PFU / feather, the negative control group was inoculated with the same volume of normal saline in the same way. On the 0d, 7d, 14d, 21d, 28d, 35d, 42d, 49d, 56d, and 63d after immunization, blood samples were taken from all test chickens under the wings and the serum was separated in time, and then the CAV antibody detection kit (purchased from IDEXX company, Antibody titer is negative lower than 999, and its detection CAV antibody highest titer value is 8861) detects the antibody titer change of test chicken, the result is as follows Figure 9 . The results showed that the anti-CAV antibody produced by the rFPV-VP1-VP2 recombinant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com