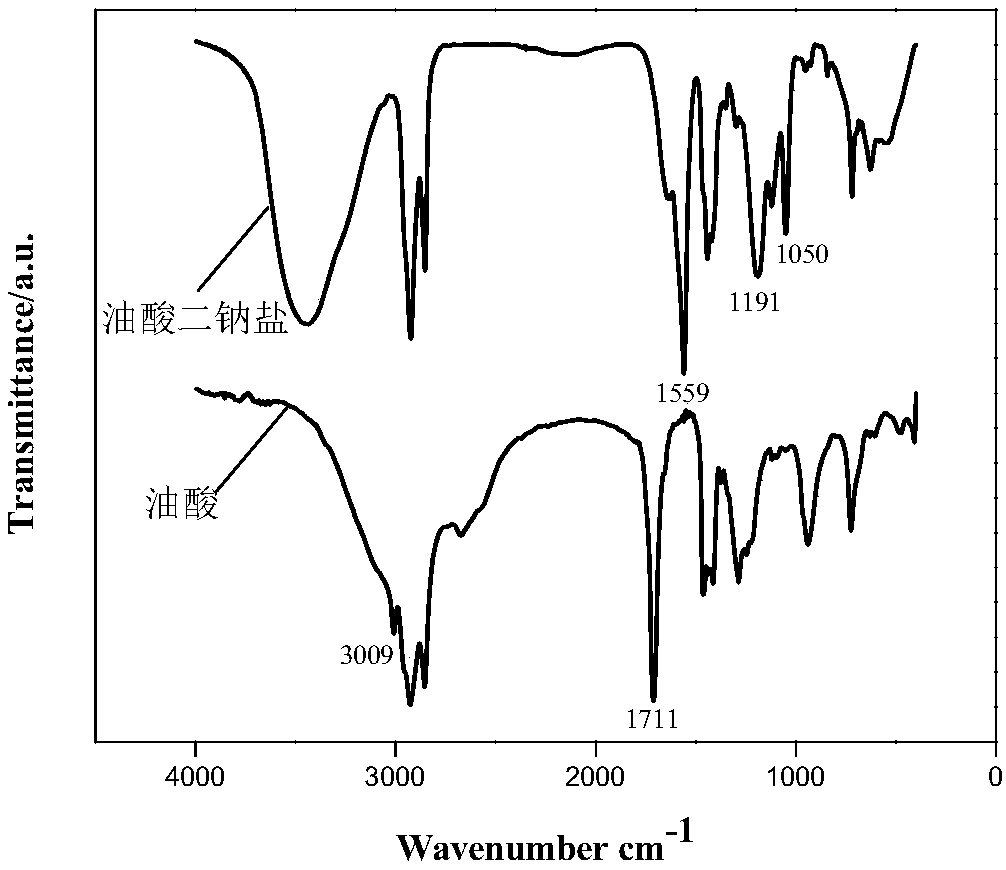
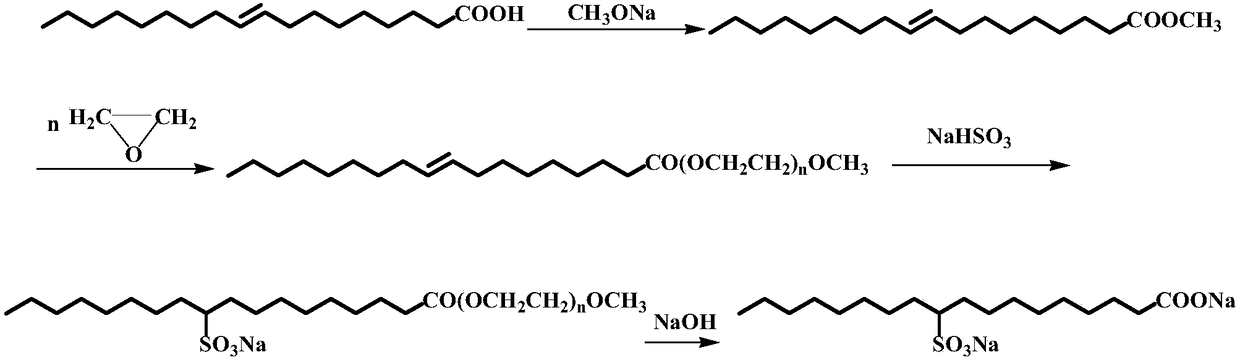
Synthesis method of disodium oleate
A technology of disodium oleic acid and synthesis method, which is applied in the field of synthesis of disodium oleic acid, can solve the problems of changing hydrophilicity and hydrophobicity, long reaction time, low mass transfer efficiency, etc., achieves changing hydrophilicity and hydrophobicity, and improving sulfonation rate and yield, solvents are cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 200g (0.708moL) of oleic acid, 46.2g (1.442moL) of anhydrous methanol and 10.02g of p-toluenesulfonic acid into a four-neck flask, stir at 65°C for 2 hours under reflux, and add sodium methoxide after the reaction to neutralize those not involved in the reaction p-toluenesulfonic acid, then wash with deionized water to remove excess salt, and finally carry out vacuum distillation to remove excess methanol to obtain methyl oleate; in the autoclave, add 100g methyl oleate and 1g catalyst, pass Inject nitrogen to replace the air in the reactor, then raise the temperature to 140°C and introduce 5g of ethylene oxide for the first time, and induce the reaction at a pressure of 0.3MPa, and then slowly introduce 215g of epoxy into the reactor after the pressure drops to 0.1MPa Ethane, when the pressure is constant and the reaction is complete, nitrogen gas is introduced when the temperature in the reactor drops to 70°C, and 320g of methyl oleate ethoxylate is exported; 51.7g...
Embodiment 2
[0028] Add 200g (0.708moL) oleic acid, 34.0g (1.062moL) anhydrous methanol and 4.00g p-toluenesulfonic acid into a four-neck flask, stir for 3 hours under reflux at 60°C, add sodium methoxide after the reaction to neutralize those not involved in the reaction p-toluenesulfonic acid, then wash with deionized water to remove excess salt, and finally carry out underpressure distillation to remove excess methyl alcohol to obtain methyl oleate; in the autoclave, add 100g methyl oleate and 1.5g catalyst, Pass nitrogen to replace the air in the reaction kettle, then raise the temperature to 120°C and introduce 3g of ethylene oxide for the first time, and carry out induction reaction at a pressure of 0.35MPa. After the pressure drops to 0.1MPa, slowly introduce 260g ring Oxyethane, until the pressure is constant and the reaction is complete, feed nitrogen when the temperature in the reactor drops to 80°C, and export 363g of methyl oleate ethoxylate; add 50.0g of methyl oleate ethoxylat...
Embodiment 3
[0030]Add 200g (0.708moL) oleic acid, 90.7g (2.832moL) anhydrous methanol and 8.00g p-toluenesulfonic acid into a four-neck flask, stir for 4 hours under reflux at 65°C, and add sodium methoxide to neutralize those not involved in the reaction p-toluenesulfonic acid, then wash with deionized water to remove excess salt, and finally carry out underpressure distillation to remove excess methyl alcohol to obtain methyl oleate; in the autoclave, add 100g methyl oleate and 1.5g catalyst, The air in the reaction kettle was replaced by nitrogen gas, then the temperature was raised to 140°C, and 8g of ethylene oxide was introduced for the first time, and the induction reaction was carried out at a pressure of 0.3MPa. After the pressure dropped to 0.1MPa, 227g ring Oxyethane, until the pressure is constant and the reaction is complete, feed nitrogen when the temperature in the reactor drops to 40°C, and export 335g of methyl oleate ethoxylate; add 50.0g of methyl oleate ethoxylate (0.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com