Preparation method of chloride alkyl hydrogen silane
A technology of chloroalkylhydrosilane and chloroalkylchlorosilane, which is applied in the field of preparation of chloroalkylhydrosilane, can solve problems such as the inability to selectively reduce chloroalkylhydrosilane with chloroalkylchlorosilane, and achieve easy recycling , fast reaction rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a preparation method of chloroalkylhydrosilane. In an ether solvent, under the catalysis of a catalyst, LiH is used as a reducing agent to reduce chloroalkylchlorosilane to chloroalkylhydrosilane; wherein, the catalyst For borane, borohydride or lithium aluminum hydride.
[0034] The experimental route is as follows:
[0035]
[0036] Among them, R 1 , R 2 Represents the same or different alkyl, halogen and other groups; R C Represents a chloroalkyl group in which C-H on the alkyl group is replaced by one or more Cl.
[0037] Compared with the prior art, the preparation method of chloroalkylhydrosilane provided by the present invention adopts LiH with lower cost as reducing agent, borane, borohydride and lithium aluminum hydride as catalyst, and ether solvent as reaction Solvent, can realize the selective reduction of chloroalkylchlorosilane, only reduce silicon-chlorine bond and retain carbon-chlorine bond, prepare chloroalkylhydrosilane c...
Embodiment 1
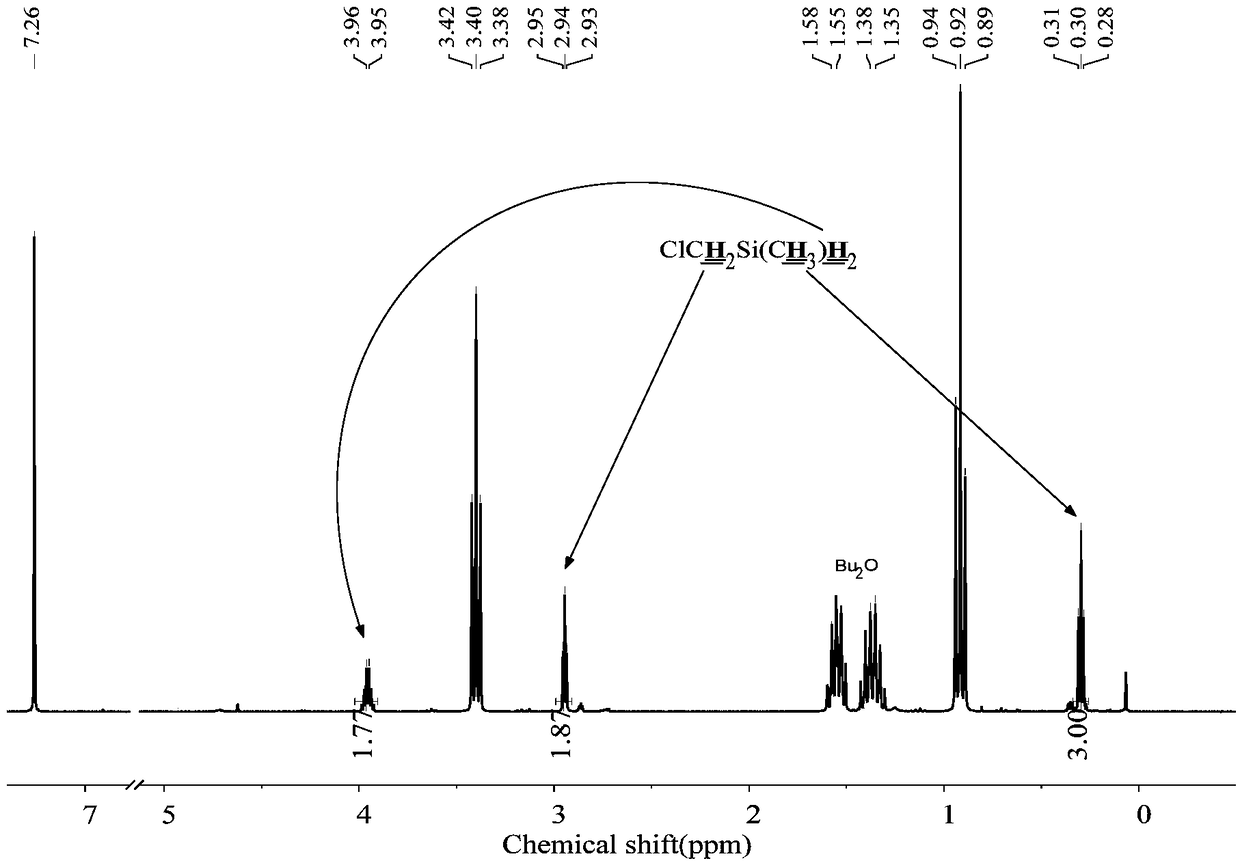
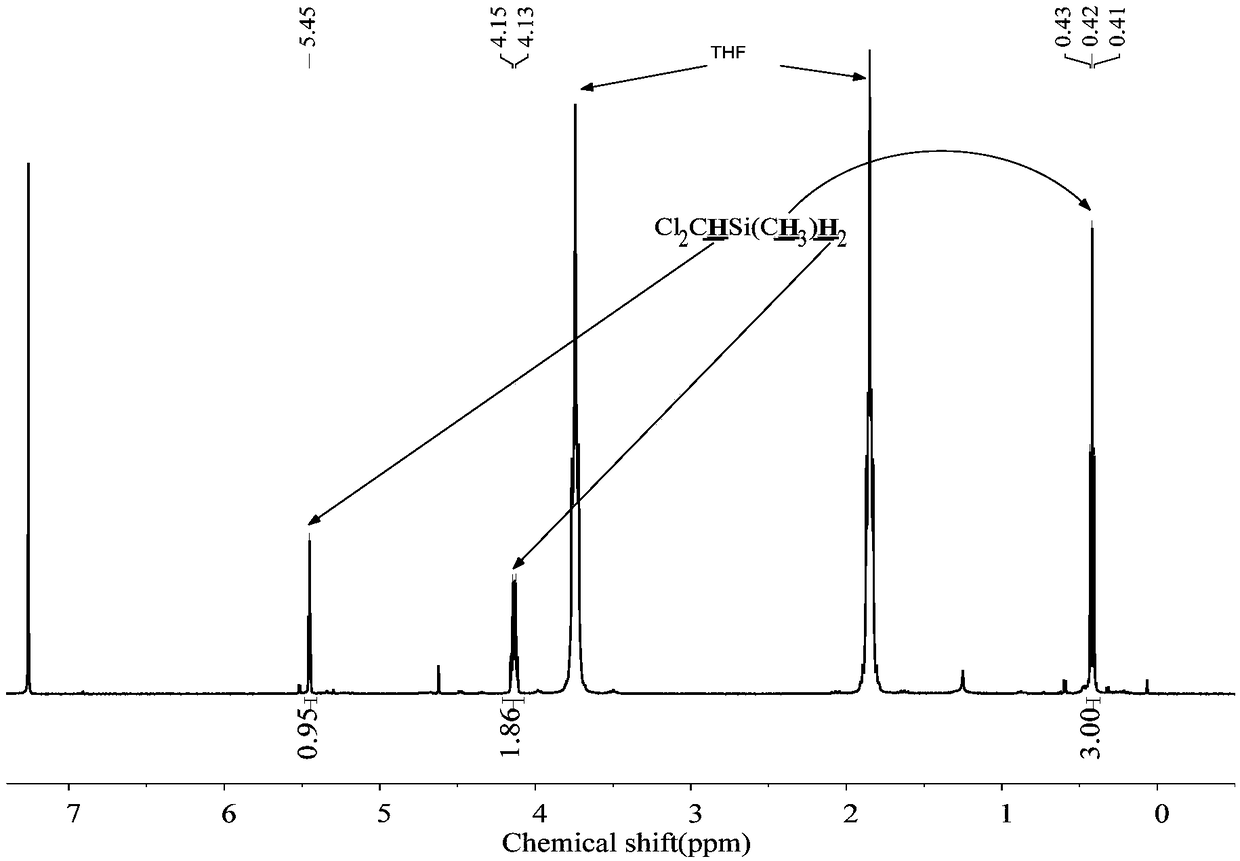
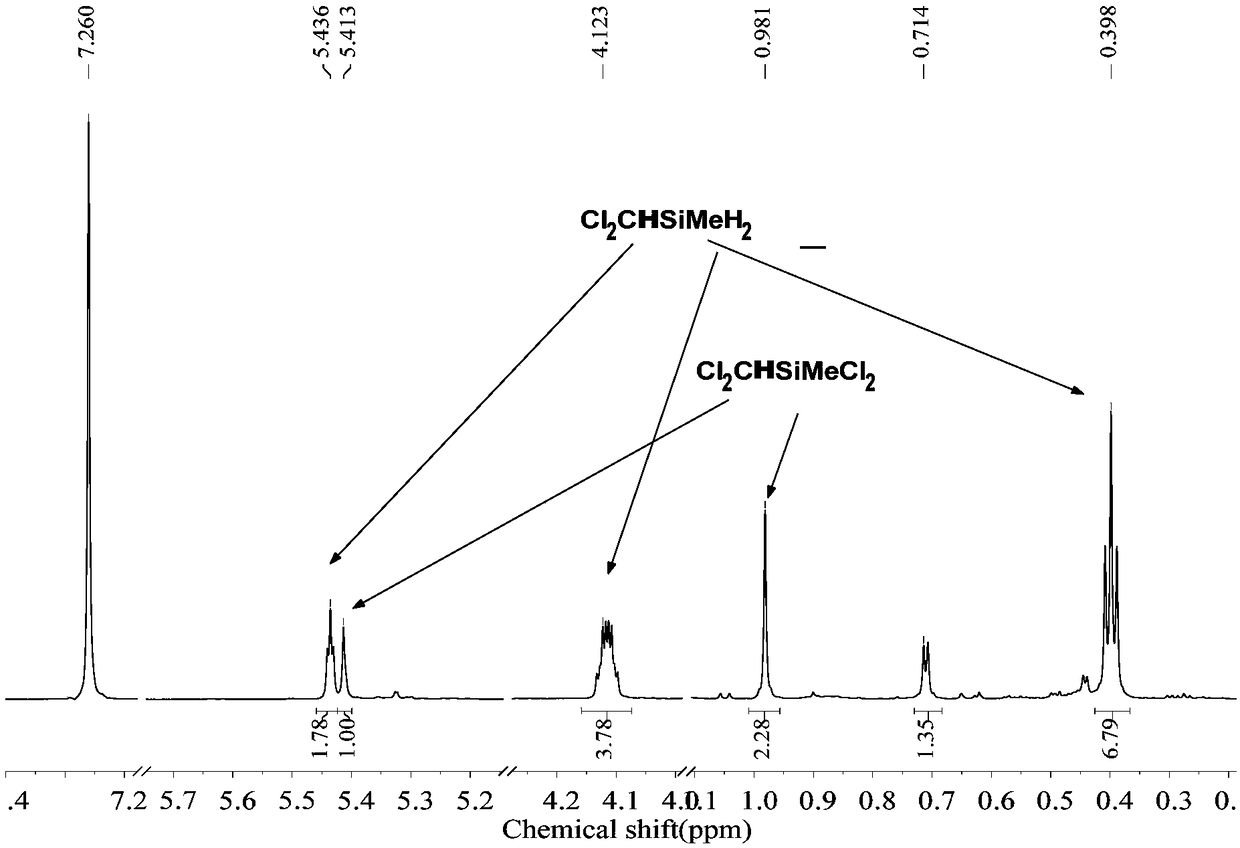
[0053] The 250mL three-neck round bottom flask was dried, connected with a spherical condenser, constant pressure funnel and gas guiding device, vacuumed three times to replace nitrogen, and at the same time baked with a hot air gun to remove attached water vapor. Under nitrogen atmosphere, add 1.8g (0.22mol) LiH, 20mL tetrahydrofuran solvent, 2ml borane dimethyl sulfide solution ((CH 3 ) 2 S·BH 3 ) (10mol / L). Add 19.2g (0.1mol) ClCH to the constant pressure funnel 2 CH 2 CH 2 SiMeCl 2 , slowly dropwise into the reaction flask under magnetic stirring. After the dropwise addition was completed, react at room temperature for 5 h to obtain the product ClCH 2 CH 2 CH 2 SiMeH 2 , the yield was 90%.
Embodiment 2
[0055] The 250mL three-neck round bottom flask was dried, connected with a spherical condenser, constant pressure funnel and gas guiding device, vacuumed three times to replace nitrogen, and at the same time baked with a hot air gun to remove attached water vapor. Under a nitrogen atmosphere, 1.8 g (0.22 mol) of LiH, 20 mL of tetrahydrofuran solvent, and 20 ml of borane tetrahydrofuran solution (1 mol / L) were added to the reaction flask. Add 17.8g (0.1mol) ClCH to the constant pressure funnel 2 CH 2 SiMeCl 2 , slowly dropwise into the reaction flask under magnetic stirring. After the dropwise addition, react at room temperature for 10 h to obtain the product ClCH 2 CH 2 SiMeH 2 , the yield was 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com