Adjuvant for desensitization vaccine and novel desensitization vaccine
A vaccine and desensitization technology, applied to allergic diseases, medical preparations containing active ingredients, allergen antigen components, etc., can solve the problems of inability to induce immune tolerance, and achieve rapid induction of peripheral immune tolerance, Rapid desensitization effect, enhanced effect of anti-allergy immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The desensitization vaccine formula in Example 1 contains glucocorticoid (dexamethasone acetate) and egg white albumin. Applying the vaccine locally to the skin can inhibit the production of inflammatory cytokines in skin cells. Such as figure 1 Shown are the levels of pro-inflammatory cytokines produced by the skin at the site of application. In Example 1, the administration of the desensitization treatment group contained egg white albumin and glucocorticoid; the administration of the standard treatment group only contained egg white albumin; the administration of the untreated group was physiological saline.
[0051] The experiments of Example 1 used 6 to 8 week old female Balb / c mice. The abdominal skin of the mice was shaved and rested for 24 hours. For mice in desensitization treatment group, apply glucocorticoid (0.1% ointment) to the depilated skin; the next day, apply egg white albumin liquid to the surface layer of skin at the same site with a cotton ball - ...
Embodiment 2
[0056] Example 2 establishes dust mite allergy and desensitization treatment models in mice.
[0057] Six to eight week old female C57BL / 6 mice (Charles River, Wilmington, MA) or Balb / c mice (Charles River) were used in this experiment. The mouse allergy model involves two steps: sensitization and challenge. The sensitization step was to intraperitoneally inject 50 μg of house dust mite extract (adsorbed on the surface of 1 mg of aluminum hydroxide) to mice. The injection volume is 0.5 ml. Attack virus after 14 days. The challenge step is to spray 10 μg HDM (HDM is dust mite extract) into the lungs of the mouse trachea. 48 hours in repeated attack once. Twenty-four hours after the second challenge, mice were euthanized using carbon dioxide. Bronchoalveolar lavage fluid (BALF) was collected for cell counting and cytokine analysis, and lung tissue was collected for section staining.
[0058] After the death of the mice, the lungs were flushed 3 times with 1 ml of sterile s...
Embodiment 3
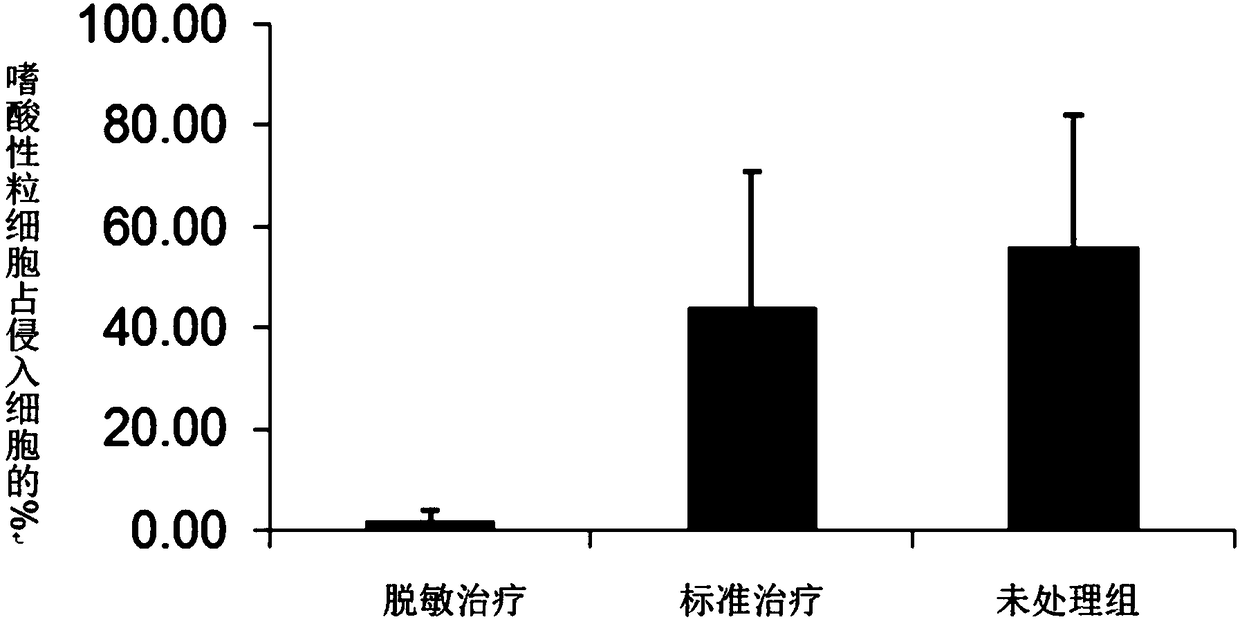
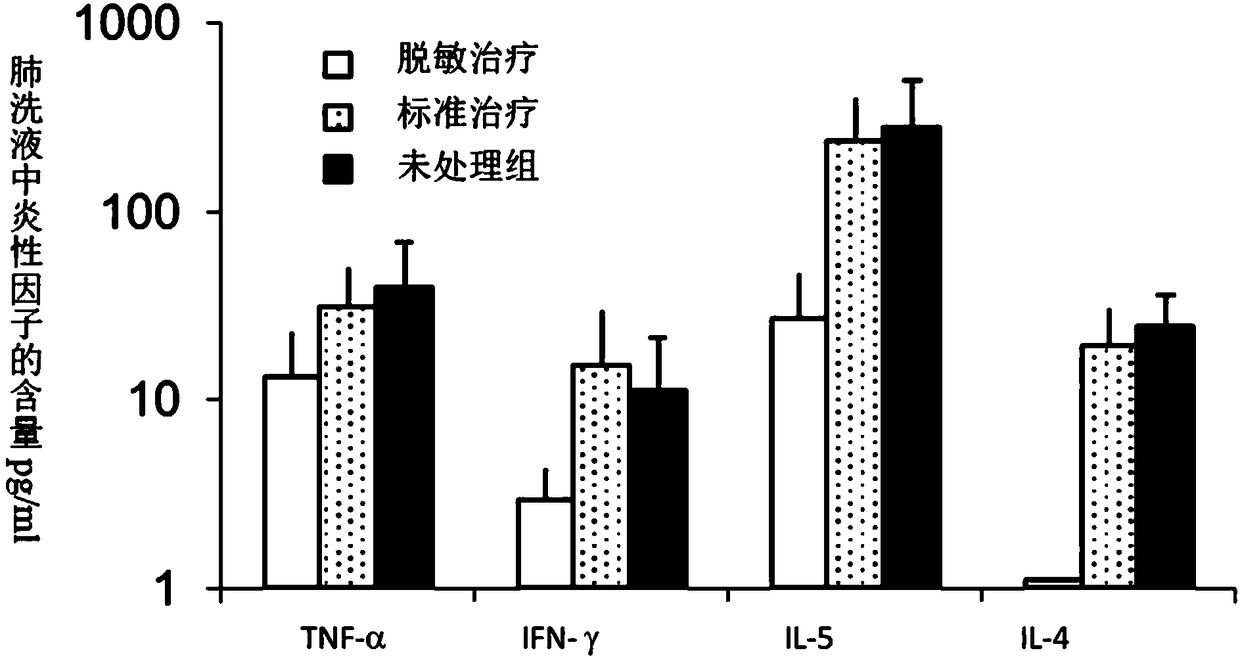
[0061] In Example 3, the desensitization vaccine containing glucocorticoids and house dust mite extract was used to treat the allergic symptoms of the sensitized mice obtained in Embodiment 2 by immunotherapy, which effectively reduced the respiratory inflammation of the mice induced by dust mite. Such as figure 2 Shown is the effect of desensitization treatment on pulmonary invading eosinophils in a mouse model of dust mites. The administration of desensitization treatment group contained dust mite extract allergen and glucocorticoid, the administration of standard treatment group only contained dust mite extract, and the administration of untreated group was normal saline. Using a desensitizing vaccine containing glucocorticoids and standardized extracts of house dust mite, immunotherapy reduced airway inflammation in mice, especially the reduction in the number of eosinophils in the lungs. image 3 Shown is the effect of desensitization treatment on cytokines in the lung ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com