Compound immunological adjuvant and vaccine
An immunoadjuvant and compound technology, applied in antiviral agents, pharmaceutical formulations, viral antigen components, etc., can solve the problem of unsatisfactory adjuvant effect, achieve obvious adjuvant effect, improve cellular immune response, and improve humoral immune response. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
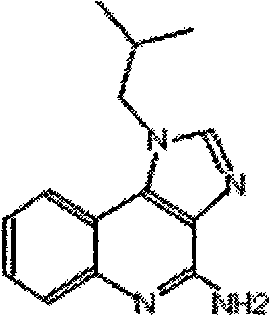
[0017] Example 1 Preparation of vaccine using gonadotropin-releasing hormone (abbreviated as GnRH) synthetic peptide as immunogen
[0018] The antigen is GnRH synthetic peptide (for castration of animals), which was synthesized by Gill Biochemical (Shanghai) Co., Ltd. The synthetic peptide sequence is: Th-GG-QHWSYGLRPGQHWSYGLRPGQHWSYGRPGQHWSYGLRPG.
[0019] Imiquimod was purchased from Wuhan Risheng Technology Development Co., Ltd.
[0020] The preparation process of compound immune adjuvant and vaccine is as follows:
[0021] (1) Preparation of CpG aqueous phase solution: prepare CpG into a solution with a concentration of 2.0 mg / mL with double distilled water, add Tween-80 until the volume concentration reaches 4%, filter and sterilize through a 0.22 μm filter membrane to obtain the aqueous phase solution;
[0022] (2) Preparation of imiquimod oil phase solution: add 1.0 mg of imiquimod to each mL of white oil to prepare an imiquimod white oil solution with a concentratio...
Embodiment 2
[0026] Example 2 Immunization test on mice using GnRH synthetic peptide vaccine as immunogen
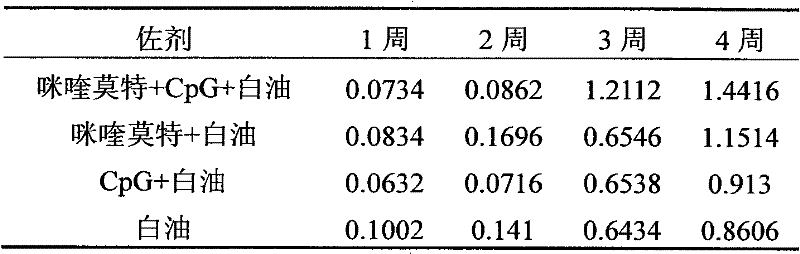
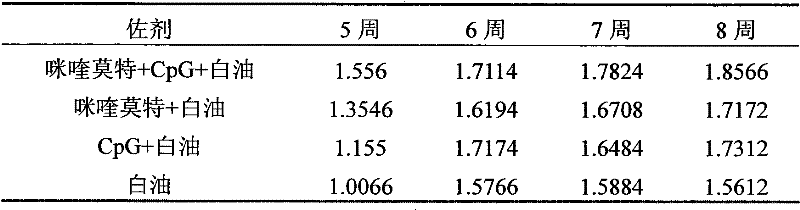
[0027] Referring to Example 1, at the same time, under the conditions of constant antigen dose and volume (insufficient part is supplemented with normal saline), respectively prepare imiquimod+white oil, CpG+white oil, single white oil as adjuvant Three vaccine control groups.
[0028] The vaccines prepared in Example 1 and the three control groups were used for group immunization respectively.
[0029] Immunization method: Male mice (18-20 g) aged 3-4 weeks were randomly divided into 4 groups, 10 mice in each group. The immunization dose is 200 μl / only. The immunization method is as follows: first immunization at the age of 28 days, and then booster immunization with the same dose at the age of 56 days.
[0030] Sampling and processing: Blood was collected once a week after immunization, and the blood sample was first placed at 37°C for 2 hours, then overnight at 4°C, centrifuged...
Embodiment 3
[0037]Example 3 Immune Test of Vaccine Using Newcastle Disease IV Inactivated Antigen as Immunogen
[0038] Using the same method as in Example 1, prepare a vaccine with Newcastle disease IV inactivated antigen, the difference is that the prepared CpG concentration in the aqueous phase solution is 1.0 mg / mL, and the imiquimod concentration in the oil phase solution is 0.5 mg / mL, the antigen adopts chicken Newcastle disease IV inactivated antigen (the virus titer before inactivation is 10 8.5 EID 50 / 0.1mL). Using inactivated Newcastle disease Ⅳ antigen as the immunogen, four groups of vaccines were prepared with the following adjuvants: imiquimod+CpG+white oil, imiquimod+white oil, CpG+white oil, and white oil. The 28-day-old chicks were immunized with four groups of vaccines (the titer of Newcastle disease maternal antibody was <2.0 Log 2), blood was collected at 2 weeks, 3 weeks and 4 weeks after immunization, the serum was separated, and the hemagglutination inhibition (H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com