Schistosoma japonica and tetanus bivalent oral or nose-dripping vaccine
A technology of host bacteria and expression vectors, applied in the field of vaccines, can solve problems such as inability to meet the needs of vaccines, and achieve the effect of improving immune protection and promoting enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
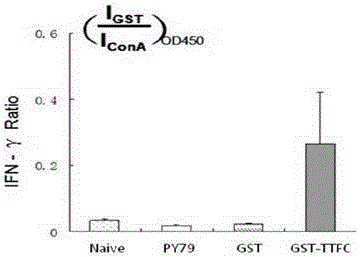
Image
Examples
Embodiment 1
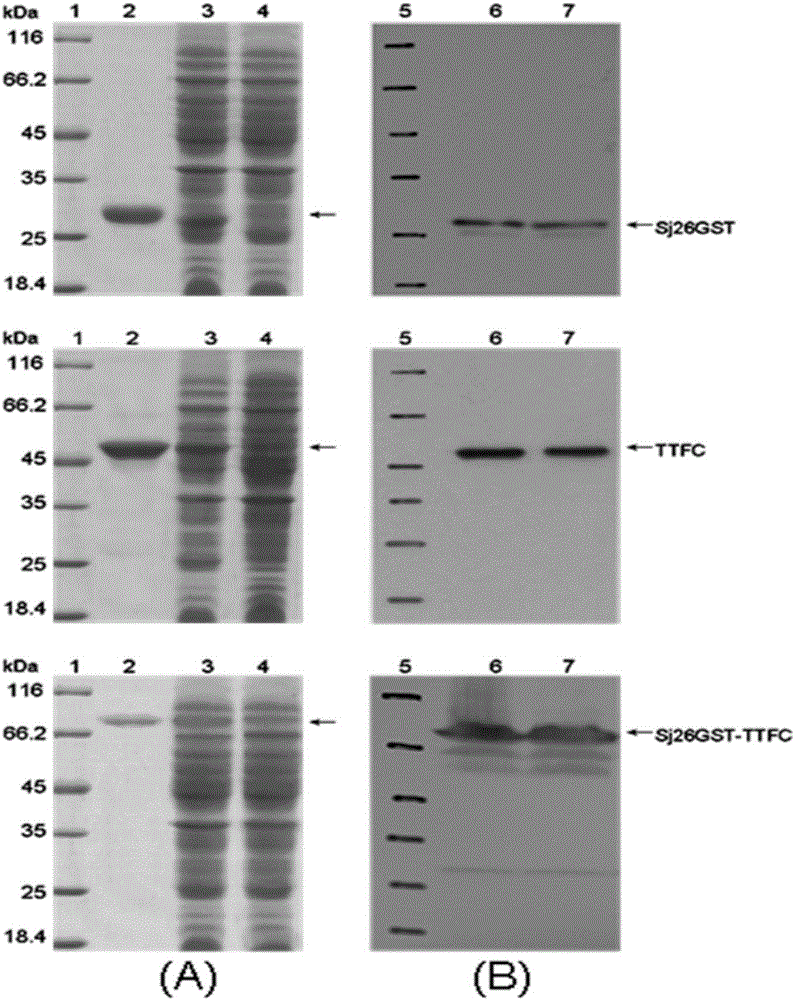
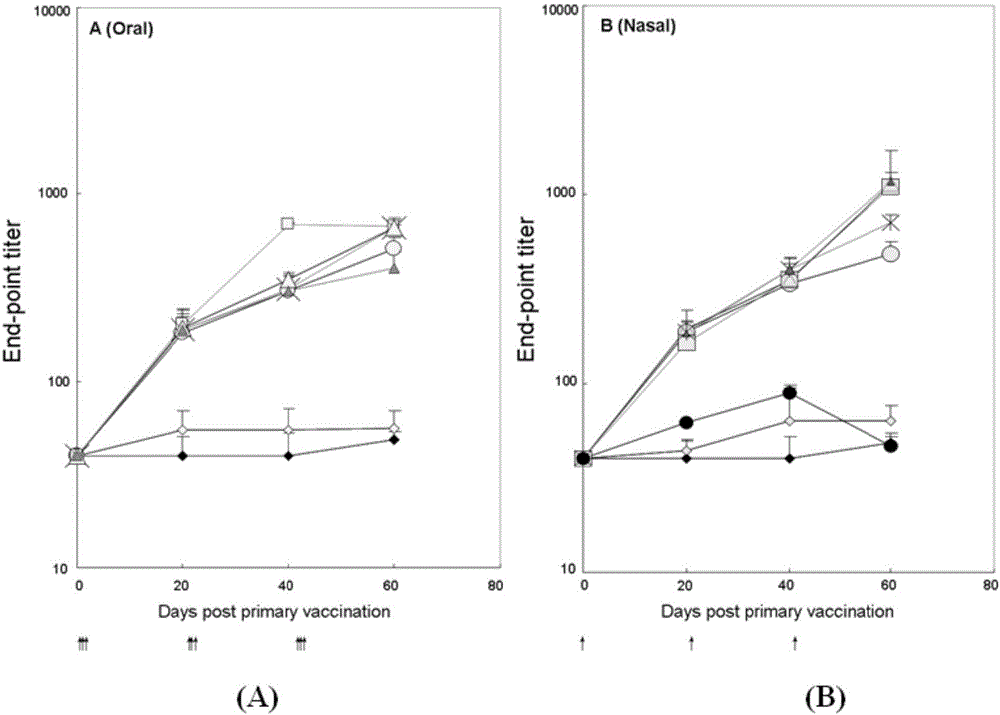
[0058] The inventors of the present application used the Schistosoma japonicum 26kDa GST (Sj26GST) protein as a model antigen in the previous research, and used the Bacillus subtilis expression plasmid pUS186 to construct the Sj26GST gene in the downstream of the Bacillus subtilis capsid gene CotC promoter and its coding sequence, at WB600 The exogenous protein Sj26GST was highly expressed on the surface of spore capsid in the extracellular enzyme-deficient strain.
[0059] Using the Bacillus subtilis shuttle integration plasmid pDG1664, CotC-Sj26GST-TTFC was integrated into the chromosome of Bacillus subtilis through double crossover replacement, and fused with CotC to express on the outer surface of the spore capsid; at the same time, the CotB-peptide linker was integrated using the Bacillus subtilis shuttle integration plasmid pDG364 - IL-2 is integrated into the chromosome of Bacillus subtilis through double crossover replacement, and is fused with CotB to express on the ou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com