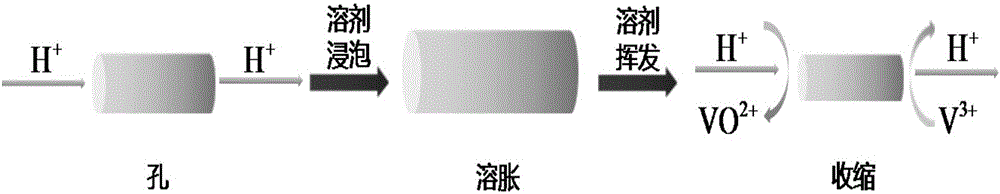
Solvent processing method for polymer porous ion conducting membrane for flow battery
An ion-conducting membrane and processing method technology, applied in fuel cells, regenerative fuel cells, circuits, etc., can solve problems such as reduced proton conductivity, and achieve the effects of improving performance, easy large-scale application, and good battery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
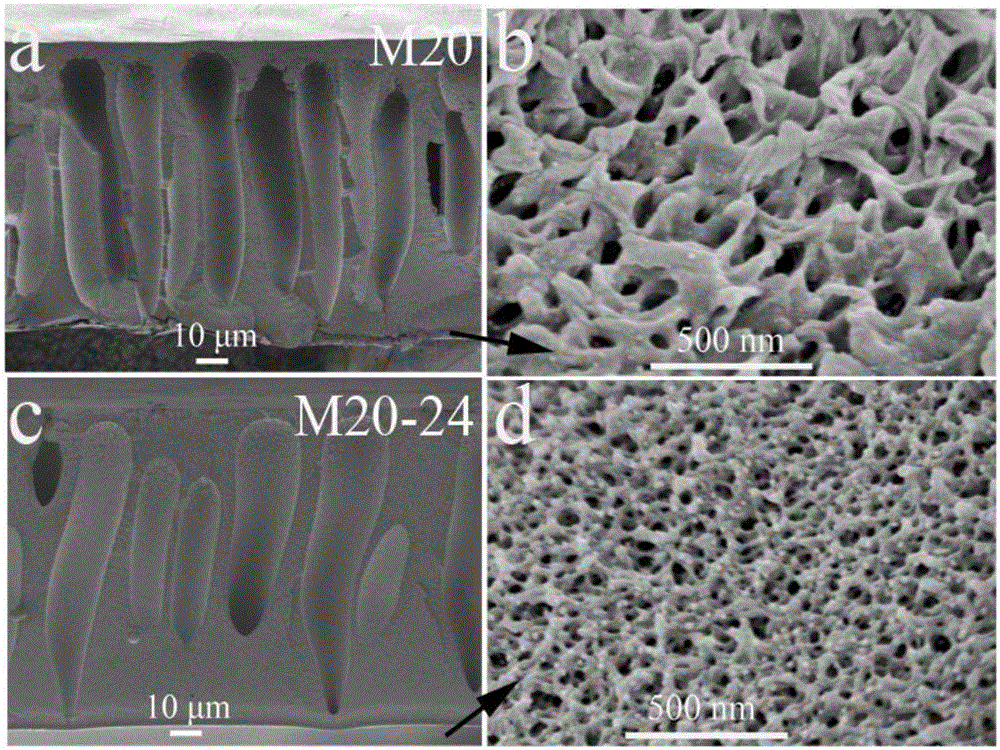
[0040]Dissolve 84g of polyethersulfone and 21g of polyvinylpyrrolidone in 195g of DMAc, stir mechanically for 24 hours to form a homogeneous polymer solution, let stand at 20°C for 2 hours to remove air bubbles in the solution, and evenly coat the casting solution on the crystal Dried glass plates were immersed in non-solvent water. Since the solubility of polyethersulfone in DMAc is much greater than that in water, water and DMAc will exchange with each other, and polyethersulfone will change from a gel state to a solid state. At the same time, the hydrophilic polyvinylpyrrolidone is dissolved in water to obtain a membrane with a porous structure. From figure 2 a It can be seen that the porous ion-conducting membrane prepared by the phase transition method has a typical asymmetric porous structure, and the cross-sectional morphology of the prepared membrane is as follows figure 2 As shown in a-b (the film thickness is about 115μm, the pore size is 2.21nm, from figure 2 ...
Embodiment 2
[0046] Dissolve 73.5g of polyethersulfone and 31.5g of polyvinylpyrrolidone in 195g of DMAc, stir mechanically for 24 hours to form a uniform polymer solution, let it stand at 30°C for 2 hours to remove air bubbles in the solution, and evenly coat the casting solution Cover on crystal-dried glass plates and immerse in non-solvent water. Since the solubility of polyethersulfone in DMAc is much greater than that in water, water and DMAc will exchange with each other, and polyethersulfone will change from a gel state to a solid state. At the same time, the hydrophilic polyvinylpyrrolidone is dissolved in water to obtain a membrane with a porous structure. Soak the prepared film in isopropanol for about 35 minutes, place it at room temperature to volatilize the isopropanol for 24 hours, and then soak it in water for more than 2 hours for use.
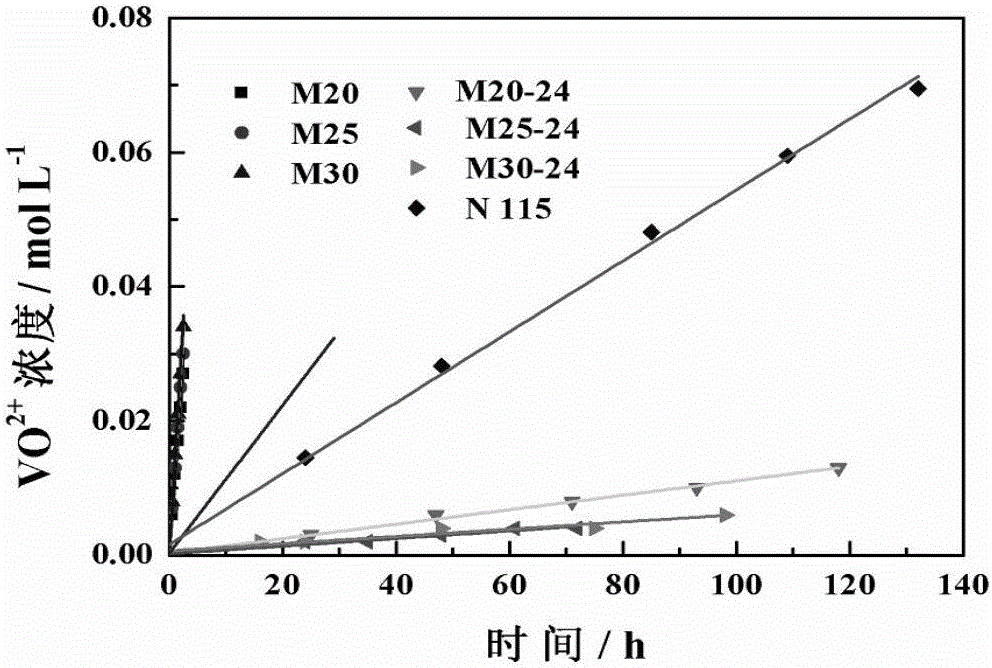
[0047] The solvent-treated membrane (thickness of about 115μm, pore size of 1.57nm, an asymmetric porous structure) was assembled into an...
Embodiment 3
[0049] Dissolve 78.75g of polyethersulfone and 26.25g of polyvinylpyrrolidone in 195g of DMAc, stir mechanically for 24 hours to form a homogeneous polymer solution, let stand at 25°C for 2 hours to remove air bubbles in the solution, and evenly coat the casting solution Cover on crystal-dried glass plates and immerse in non-solvent water. The solvent and non-solvent exchange each other to obtain a membrane with a porous structure. Soak the prepared film in isopropanol for about 35 minutes, place it at room temperature to let the isopropanol evaporate for 24 hours, and soak it in water for more than 2 hours after the solvent evaporates.
[0050] The solvent-treated membrane (thickness of about 115μm, pore size of 1.30nm, asymmetric porous structure) is assembled into an all-vanadium redox flow battery, in which the catalytic layer is activated carbon felt, the bipolar plate is a graphite plate, and the effective area of the membrane is 48cm 2 , with a current density of 80m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com