Levo-S-oxiracetam granule with good content uniformity and preparation method thereof
A technology of uniformity and granulation, which is applied in the field of levo-oxiracetam granules and its preparation, can solve the problems of difficult control of particle size, strong hygroscopicity of granules, easy adhesion and agglomeration, etc., and achieve simple and feasible preparation process and uniform granule content Good performance, not easy to absorb moisture and agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A levoxiracetam granule with good content uniformity is prepared according to the following steps:
[0026]
[0027] Preparation process:
[0028] 1. Pretreatment of raw and auxiliary materials: take the prescribed amount of levoxiracetam and dissolve it in the prescribed amount of ethanol solution to obtain levoxiracetam ethanol solution, and set aside; take L-cysteine, mannitol, and microcrystalline fiber in addition Sodium carboxymethyl cellulose, lactose, and sorbitol are placed in a universal pulverizer, pulverized through a 100-mesh sieve, and set aside;
[0029] 2. Granulation: place the mixed powder of the excipients obtained in the pretreatment and ethanol solution of levoxiracetam in a wet granulator, add starch slurry, start the granulator (install 18-mesh nylon sieve), and start granulation;
[0030] 3. Drying: Put the wet granules into the fluidized bed, set the temperature of the hot bed at 50°C to 70°C, and start drying; observe the boiling and blasting...
Embodiment 2
[0083] A levoxiracetam granule with good content uniformity is prepared according to the following steps:
[0084]
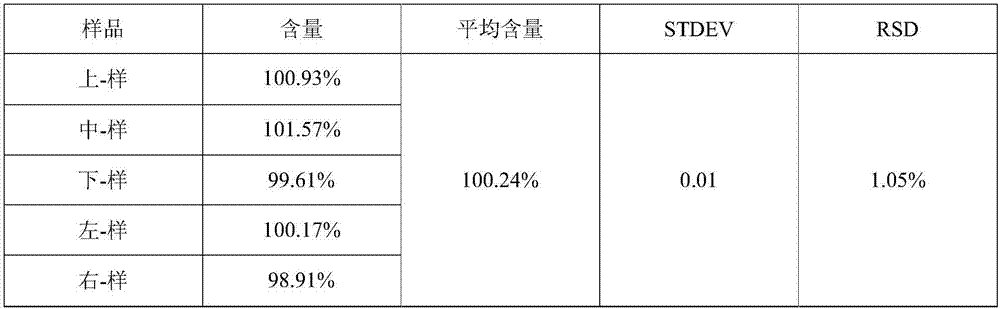
[0085] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and Hughes angle is lower than 36 °, and the content uniformity test result shows that this product content uniformity is good, each after its total mixing The content RSD of point granules is less than 1%. The difference test of the filling volume shows that the loading volume of this product is less than ±4%. The loading volume of this product is stable and controllable. The increase is small, and the relevant substances in the preparation process only increase by 0.02%. The stability test results show that the sample quality is stable in six months after acceleration, and stable in 24 months in a long-term period, so the valid...
Embodiment 3
[0087] A levoxiracetam granule with good content uniformity is prepared according to the following steps:
[0088]
[0089] Preparation process: prepared according to the preparation process of Example 1. Carry out the test by the test method of embodiment 1, the Hughes angle test measurement result shows that this product granule fluidity is good, and Hughes angle is lower than 37 °, and the content uniformity test result shows that this product content uniformity is good, each after its total mixing The content RSD of point granules is less than 2%. The difference test of the filling volume shows that the loading volume difference of this product is less than ±5%. The loading volume of this product is stable and controllable. The increase is small, and the related substances only increase by 0.03% in the preparation process. The stability test results show that the quality of the sample is stable in six months after acceleration, and the quality is stable for 24 months in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com