A preparation of yeast cell wall particles that promotes the oral absorption of protein and polypeptide drugs
A yeast cell wall and drug technology, which is applied in drug combinations, peptide/protein components, medical preparations of non-active ingredients, etc., can solve problems such as unpredictable development prospects, large protein molecular weight, and inability to achieve therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
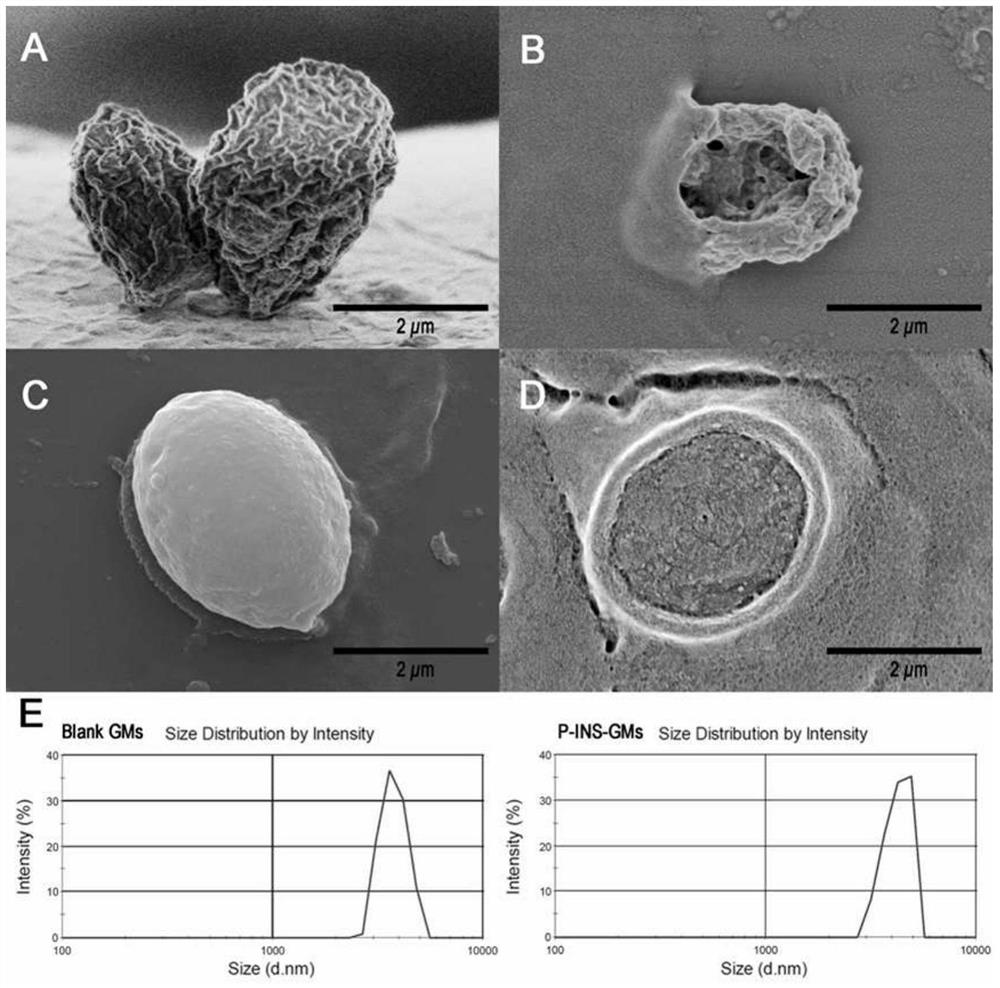
[0031] Preparation of yeast cell wall granule powder: take 100g of baker's yeast, add 1L of NaOH (1M) solution, incubate in a constant temperature water bath at 80°C for 1h, and stir slowly at 50rpm. Then centrifuge at 2000g for 10min to collect the precipitate, redisperse the precipitate in 1L pure water, adjust the pH to about 4.5 with 2M hydrochloric acid, incubate in a constant temperature water bath at 55°C for 1h under slow stirring at 50rpm, and centrifuge again at 2000g for 10min to collect the precipitate. Wash with 1L pure water once, wash four times with 200mL isopropanol, wash twice with 200mL acetone, and finally collect the precipitate, dry it in vacuum, weigh and calculate the yield;
[0032] Encapsulated insulin: Weigh 1g of prepared yeast cell granules (GMs) powder and disperse in 5mL insulin (INS) solution (10mg / mL), vortex to mix evenly, adjust the pH to 2 with 1M hydrochloric acid, and then incubate at 4°C Under the condition of 200rpm magnetic stirring for...
Embodiment 2
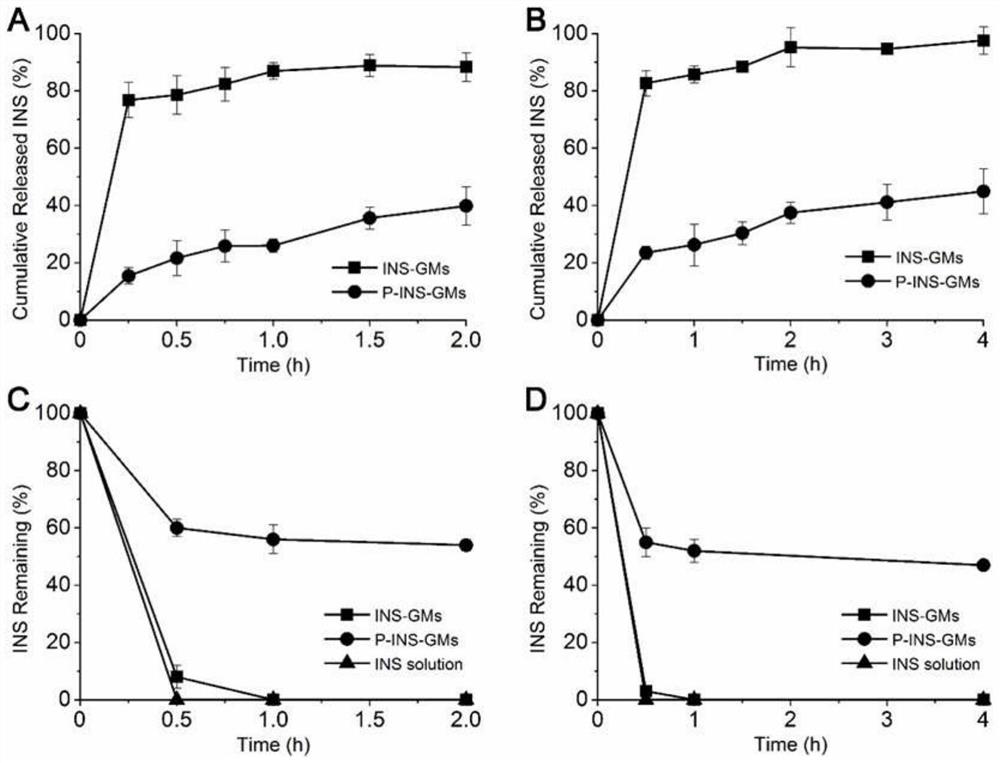
[0034] Example 2 In vitro Release and Enzymatic Degradation Resistance of Insulin-loaded Yeast Cell Wall Microparticles
[0035] In vitro release test: Prepare 10mL of enzyme-free artificial gastric juice or artificial intestinal juice, preheat it to 37°C in a constant temperature water bath shaker, take an appropriate amount of freeze-dried insulin yeast cell wall particles (P-INS-GMs), add to the enzyme-free In artificial gastric juice or artificial intestinal juice, 200 μl samples were taken at 0.25, 0.5, 0.75, 1, 1.5 and 2 hours and an equal amount of preheated artificial gastric juice or artificial intestinal juice without enzymes was added. The sample was centrifuged at 8000 g and passed through a 0.25 μm filter membrane After injection, calculate the released INS content, and its release curve is shown in figure 2 shown;
[0036] Anti-enzyme degradation test: Prepare 10mL of artificial gastric juice or artificial intestinal juice, preheat it to 37°C in a constant temp...
Embodiment 3
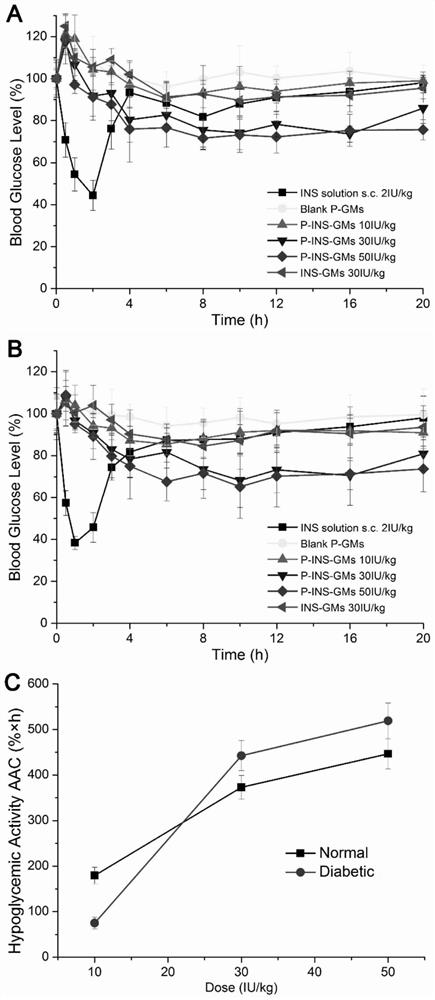
[0037] Example 3 Oral Hypoglycemic Experiment of Insulin-loaded Yeast Cell Wall Microparticles
[0038] Normal rats and diabetic rats were randomly divided into 6 groups, 4 rats in each group, fasted for 12 hours before the experiment, free to drink water, the preparations of the 6 groups were P-INS-GMs (10, 30 and 50IU / kg), uncured yeast cell wall particles INS-GMs (30IU / kg), blank internal poloxamer gelled yeast cell wall particles P-GMs and INS solution group (2IU / kg), wherein, except Except for the subcutaneous injection in the INS solution group, the rest of the preparation groups were administered by intragastric administration;
[0039] At specific time points after the administration of each group of preparations, blood was taken from the tail vein of the rat, and one drop was added to the sensing area of the blood glucose meter test paper to measure the instant blood sugar value, and the average value was measured 3 times at each time point, and the measured blood s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com