9,9'-(3,3'-dihydroxy-4,4'-diphenyl ether group) difluorone reagent and its preparation method and application
A technology of double fluorescent ketone and diphenyl ether based diformaldehyde, which is applied in chemical instruments and methods, luminescent materials, chemiluminescence/bioluminescence, etc., can solve the problems of unsatisfactory sensitivity and selectivity, and achieve stable properties and low cost. , selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
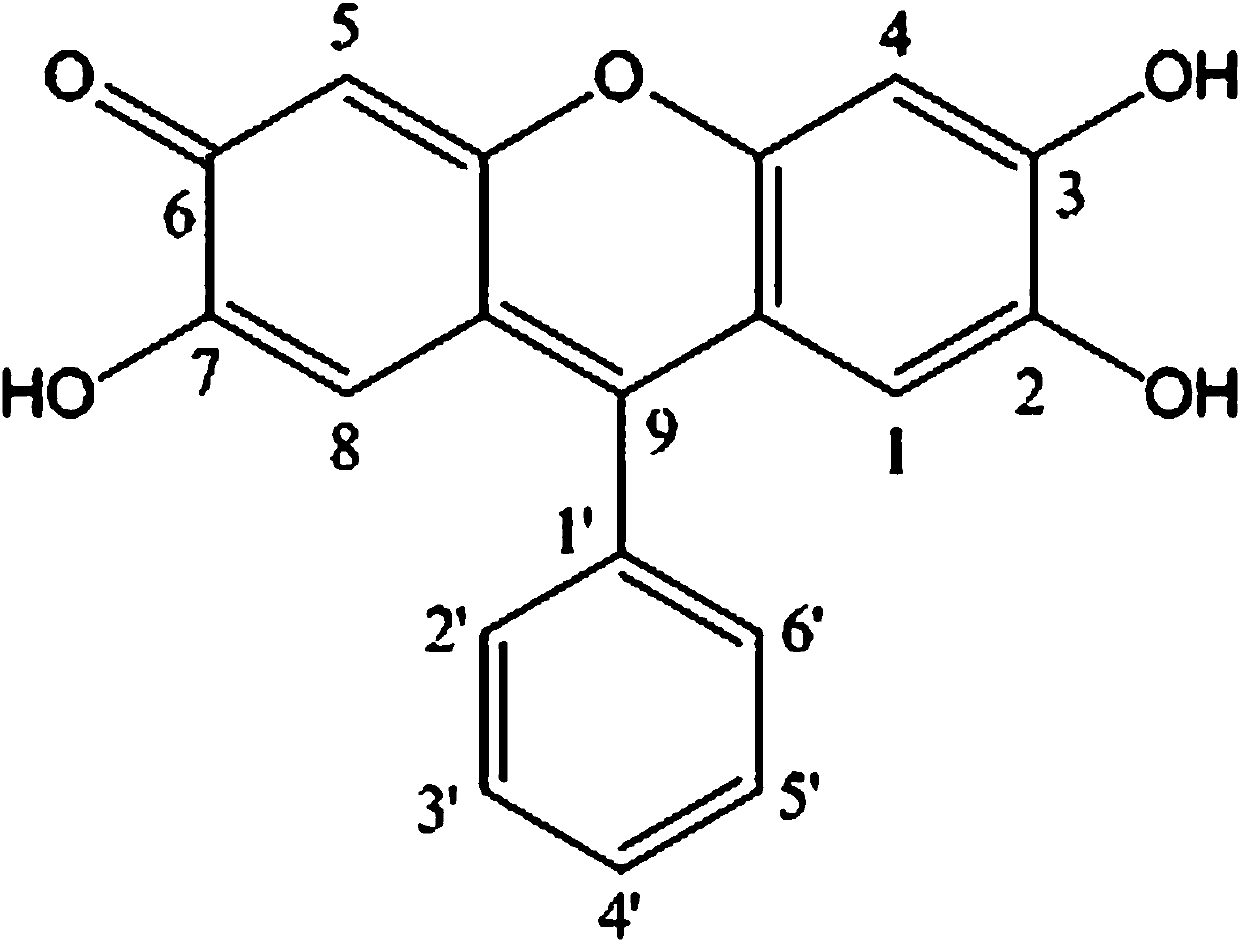
[0020] Example 1: A 9,9'-(3,3'-dihydroxy-4,4'-diphenyl ether group) difluorone reagent, referred to as 9,9'-DHDPDF, its molecular structural formula is:
[0021] .
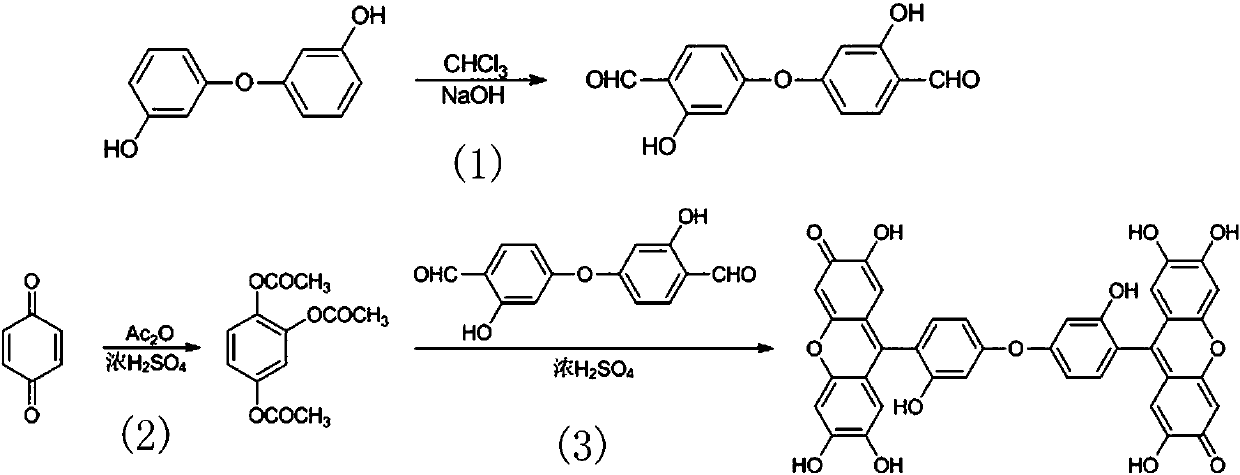
[0022] The preparation method of the 9,9'-(3,3'-dihydroxy-4,4'-diphenyl ether group) bisfluorone reagent comprises the following steps:
[0023] (1) Synthesis of trimerol triacetate: add 28 mL of acetic anhydride and 1 mL of concentrated H to a 100 mL beaker 2 SO 4 Stir and mix, add 11g of p-benzoquinone under vigorous stirring, react at 40°C-50°C for 1.5h, pour it into 100 mL of cold water under stirring, precipitate a white precipitate, obtain 22 g of crude product by suction filtration, and recrystallize from ethanol to obtain pure product Triphenol triacetate 20 g;
[0024] (2) Preparation of 3,3'-dihydroxy-4,4'-diphenyl ether dicarbaldehyde: Add 10.1g (50 mmol) 3,3'-dihydroxydicarboxylate into a 250mL flask equipped with a reflux condenser Phenyl ether, 120 mL absolute ethanol, 28 g (0.7 mol) sodium hyd...
experiment example 1
[0026] Experimental example 1: 9,9'-(3,3'-dihydroxy-4,4'-diphenyl ether group) difluorone on Co 2+ photometric analysis.
[0027] Accurately pipette ≤20 μg of Co 2+ In a 25 mL volumetric flask, add 2.0 mL of borax-sodium hydroxide buffer solution with pH 9.0, 10 g / L cetylpyridinium bromide (CPB) microemulsion (CPB: n-butanol: n-heptane : water = 1.0: 1.0: 1.0: 97) 3.0 mL, 0.2 g / L 9,9'-DHDPDF 1.0 mL, then dilute to the mark with twice distilled water, shake well and let stand for 10 min, use a 1 cm cuvette , At 660 nm, the absorbance of the complex was measured with a reagent blank as a reference.
[0028] Sample analysis: according to the literature [Journal of Pharmaceutical Analysis, 1997, Vol. 77, No. 4, 261-264. Wei Qin, Du Bin, Zhang Xuzhen], pipette vitamin B 12 Put 1.0 mL of the sample in a 50 mL beaker, add 8 mol / L nitric acid for nitration, add 5 mL of 2% hydrogen peroxide and evaporate to near dryness, add 3 mL of 1∶1 hydrochloric acid and appropriate amount of wa...
experiment example 2
[0035] Experimental example 2: Determination of nickel by 9,9'-(3,3'-dihydroxy-4,4'-diphenyl ether group) bisfluorone fluorescence quenching method.
[0036] Accurately weigh ≤0.005µg nickel in a 25ml volumetric flask, add 2 mL of 3 % Tritonx-100 aqueous solution, 3.0 mL of borax-hydrochloric acid buffer solution with pH 9.4, 2×10 -5 mol / L 9,9'-DHDPDF ethanol solution 2.0 mL, dilute to volume with water, and place for 10 min. Using a 1cm quartz cuvette, at the excitation wavelength (λ ex )465 nm, emission wavelength (λ em ) at 586 nm to measure the fluorescence intensity F of the complex, and at the same time measure the fluorescence absorbance F of the reagent blank under this condition, and calculate the fluorescence quenching value ΔF (ΔF=F 0 -F).
[0037] Sample determination: Weigh 1.0000 g of solid sample, carry out microwave digestion on the sample according to the literature [Food Science, 2011, Vol. Volume 2, pp. 158-160, Wei Qin] separated and enriched nickel, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com