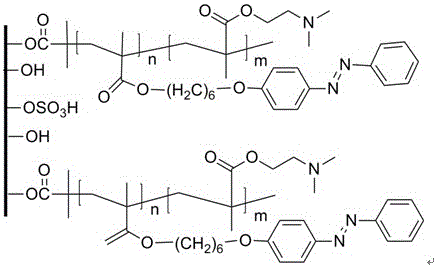
Preparation method of nanocellulose-based azobenzene-containing random polymer
A technology of nanocellulose and p-hydroxyazobenzene, which is applied in the field of preparation of random polymers containing azobenzene, can solve problems such as poor solubility, and achieve the effects of clear conditioning, clear synthesis route and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
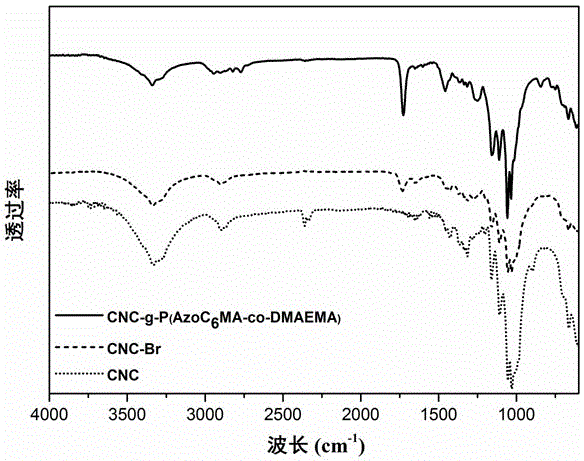
Image
Examples
Embodiment 1
[0027]Dissolve 9.9g of p-hydroxyazobenzene, 10.2g of 6-chloro-1-hexanol, 10.35g of potassium carbonate, and 0.5g of potassium iodide in 200mL of N,N-dimethylformamide. The reaction temperature of the system is 90°C, and the reaction time is for 12 hours. After the reaction was completed, it was diluted and extracted with chloroform, and the organic phase was dried and filtered with suction, then recrystallized and purified in ethanol several times, and dried in vacuum for 24 hours to obtain product A. 2.46g of product A and 5.0mL of dried triethylamine were dissolved in 50mL of tetrahydrofuran under the protection of nitrogen, and the mixed liquid was cooled to 0°C in an ice bath. Then, 2 mL of methacryloyl chloride was dissolved in 50 mL of tetrahydrofuran, and was added dropwise to the mixed liquid after ice-bathing through a constant pressure dropping funnel, and the time used for the dropwise addition was 30 minutes. After the dropwise addition, the system continued to re...
Embodiment 2
[0029] Dissolve 4.9g of p-hydroxyazobenzene, 5.1g of 6-chloro-1-hexanol, 5.18g of potassium carbonate, and 0.25g of potassium iodide in 100mL of N,N-dimethylacetamide. The reaction temperature of the system is 80°C, and the reaction time is for 24 hours. After the reaction was completed, it was diluted and extracted with chloroform, and the organic phase was dried and filtered with suction, then recrystallized and purified in ethanol several times, and dried in vacuum for 24 hours to obtain product A. 1.23g of product A and 2.5mL of dried ethylenediamine were dissolved in 25mL of dichloromethane under the protection of argon, and the mixed liquid was cooled to 0°C in an ice bath. Then, 1 mL of methacryloyl chloride was dissolved in 25 mL of dichloromethane, and was added dropwise to the mixed liquid after ice-bathing through a constant pressure dropping funnel, and the time for the dropwise addition was 1 hour. After the dropwise addition, the system was continued to react un...
Embodiment 3
[0031] Dissolve 9.9g of p-hydroxyazobenzene, 15.8g of 6-chloro-1-hexanol, 15.88g of potassium carbonate, and 0.6g of potassium iodide in 220mL of N,N-diethylformamide. The reaction temperature of the system is 100°C, and the reaction time is for 18 hours. After the reaction was completed, it was diluted and extracted with chloroform, and the organic phase was dried and filtered with suction, then recrystallized and purified in ethanol several times, and dried in vacuum for 24 hours to obtain product A. 2.46g of product A and 7.0mL of dried ethylenediamine were dissolved in 60mL of chloroform under the protection of argon, and the mixed liquid was cooled to 0°C in an ice bath. Then, 5 mL of methacryloyl chloride was dissolved in 80 mL of chloroform, and was added dropwise to the mixed liquid after ice-bathing through a constant-pressure dropping funnel, and the time used for the dropwise addition was 45 minutes. After the dropwise addition, the system continued to react under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com