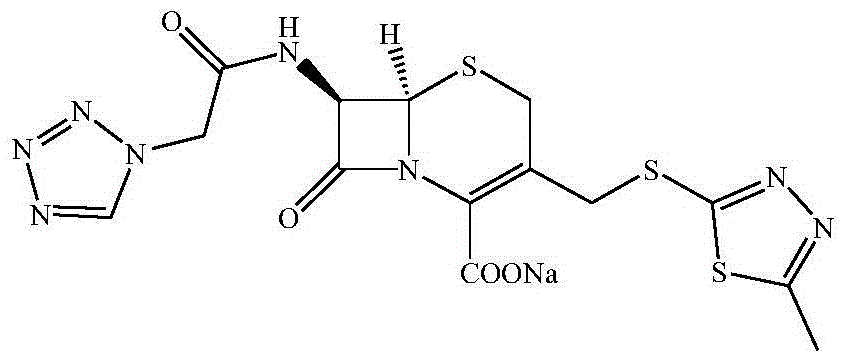
Preparation method of cefazolin sodium with previous research quality and medicine preparation of cefazolin sodium
A technology for cefazolin sodium and preparation, which is applied in the field of preparation of the original development quality cefazolin sodium, can solve the problems of cefazolin sodium solvent residue and high water content, high production cost, reduced production efficiency, etc. The effect of using freeze-drying technology to control solvent residues and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 (preparation of cefazolin acid)
[0022] Add 500g of boron trifluoride-dimethyl carbonate solution to 500g of dimethyl carbonate, add 150g of 2-mercapto-5-methyl-1,3,4-thiadiazole, 300g of 7-ACA and heat up to 35~40℃ while stirring After the reaction, add 500ml dimethylformamide, stir for 10 minutes, slowly add hydrochloric acid to pH 2~3 within 20 minutes, adjust the temperature to 25-35°C for 60 minutes, filter, and wash with acetone twice , vacuum-dried to obtain the crude product of TDA;
[0023] Preparation of mixed anhydrides from dichloromethane, tetrazoleacetic acid, triethylamine and pivaloyl chloride;
[0024] Add crude TDA to dichloromethane solvent, lower the temperature to -30°C, add tetramethylguanidine dropwise within 30 minutes, then add dropwise mixed anhydride to control the temperature and react, and obtain cefazolin acid after phase separation, extraction, crystallization and other steps crystals and then purified.
Embodiment 2
[0025] Embodiment 2 (preparation of cefazolin sodium)
[0026] Put 45.5kg of cefazolin acid and 280kg of acetonitrile into a 1000L enamel kettle at room temperature, stir, add 82kg of 5% sodium hydroxide aqueous solution to adjust PH≈7, start to cool down to -5~-10°C, let stand to separate, and acetonitrile Add 15 kg of anhydrous sodium sulfate to the layer and dry for 2 hours, detect that the water content of the acetonitrile mother liquor is 1.4%, filter out the sodium sulfate, then filter the mother liquor with a precision filter, and use a high-vacuum multi-stage Roots pump to distill the mother liquor under reduced pressure at 25-30°C, vacuum The temperature is -0.09MPa~-0.1MPa. When distilling to solid precipitation, stop the distillation, lower the temperature to 0-5°C, stir for 2h, centrifuge, and vacuum-dry the filter cake with a high-vacuum multi-stage Roots pump at a temperature of 30-5°C. 40°C, vacuum degree -0.09MPa~-0.1MPa, after drying, a total of 43.6kg of cefa...
Embodiment 3
[0027] Embodiment 3 (preparation of cefazolin sodium)
[0028] Put 45.5kg of cefazolin acid and 280kg of acetonitrile into a 1000L enamel kettle at room temperature, stir, add 41kg of 10% sodium hydroxide aqueous solution to adjust PH≈7, start to cool down to -5~-10°C, let stand to separate, and acetonitrile Add 15 kg of anhydrous sodium sulfate to the layer and dry for 2 hours, detect that the water content of the acetonitrile mother liquor is 1.6%, filter out the sodium sulfate, and then filter the mother liquor with a precision filter. The temperature is -0.09MPa~-0.1MPa. When distilling to solid precipitation, stop the distillation, lower the temperature to 0-5°C, stir for 2h, centrifuge, and vacuum-dry the filter cake with a high-vacuum multi-stage Roots pump at a temperature of 30-5°C. 40°C, vacuum at -0.09MPa~-0.1MPa, after drying, a total of 43.0kg of cefazolin sodium was obtained, yield: 90.3%, moisture: 0.68%, impurity E was 0.17%, total impurities were 0.89%, aceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com