Terbinafine hydrochloride compound sustained-release capsule and preparation method and application
A technology of terbinafine hydrochloride and sustained-release capsules, which is applied in the field of medicine, can solve problems such as fungal cell death and ergosterol deficiency, and achieve the effects of stable blood drug concentration, high effective ratio of drugs, and simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
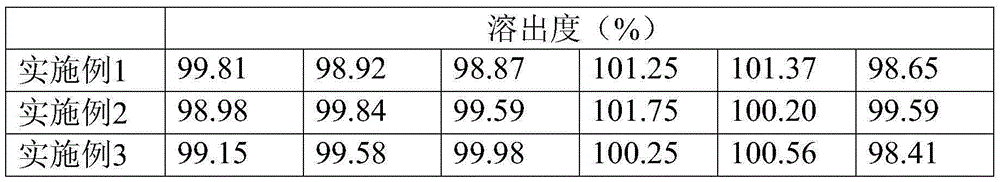
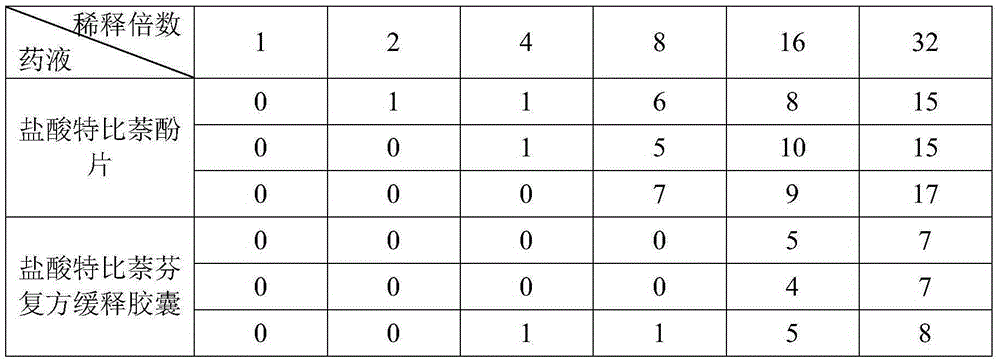
[0029] A compound sustained-release capsule of terbinafine hydrochloride. The components and parts by weight of the sustained-release capsule include: 5 parts of terbinafine hydrochloride, 50 parts of seaweed, 35 parts of Cyperus cyperi, 20 parts of Amomum chinensis, and 40 parts of licorice 30 parts of myrrh, 20 parts of herb, 35 parts of dried hemp, 35 parts of cicada slough, 25 parts of water hyacinth fruit, 20 parts of flower ant insect, 30 parts of jerky beetle, 3 parts of sucrose pill, 0.3 part of ethyl cellulose , 0.08 parts of glycerides, 0.05 parts of polyethylene glycol, 2 parts of microcrystalline cellulose, and 1 part of talcum powder.
[0030] A preparation method of terbinafine hydrochloride compound sustained-release capsules, comprising the steps of:
[0031] 1) preparing materials, preparing materials according to the components and parts by weight of a terbinafine hydrochloride compound sustained-release capsule, wherein terbinafine hydrochloride is pulverize...
Embodiment 2
[0039] A compound sustained-release capsule of terbinafine hydrochloride. The components and parts by weight of the sustained-release capsule include: 10 parts of terbinafine hydrochloride, 60 parts of seaweed, 40 parts of Cyperus cyperi, 25 parts of Amomum chinensis, and 45 parts of licorice 35 parts of myrrh, 25 parts of atractylodes atractylodes, 40 parts of dried hemp, 40 parts of cicada slough, 30 parts of water hyacinth fruit, 25 parts of flower ant insects, 35 parts of gillworms, 5 parts of sucrose pills, 0.5 parts of ethyl cellulose , 0.1 part of glyceride, 0.08 part of polyethylene glycol, 4 parts of microcrystalline cellulose, and 2 parts of talcum powder.
[0040] A preparation method of terbinafine hydrochloride compound sustained-release capsules, comprising the steps of:
[0041] 1) preparing materials, preparing materials according to the components and parts by weight of a terbinafine hydrochloride compound sustained-release capsule, wherein terbinafine hydroch...
Embodiment 3
[0049] A compound sustained-release capsule of terbinafine hydrochloride. The components and parts by weight of the sustained-release capsule include: 7 parts of terbinafine hydrochloride, 56 parts of seaweed, 39 parts of Cyperus cyperi, 21 parts of Amomum japonicus, 43 parts of licorice 34 parts of myrrh, 22 parts of herb, 36 parts of dried hemp, 38 parts of cicada slough, 29 parts of water hyacinth fruit, 23 parts of flower ant insect, 34 parts of jerky beetle, 4 parts of sucrose pill, 0.4 part of ethyl cellulose , 0.09 parts of glycerides, 0.06 parts of polyethylene glycol, 3 parts of microcrystalline cellulose, and 1.2 parts of talcum powder.
[0050] A preparation method of terbinafine hydrochloride compound sustained-release capsules, comprising the steps of:
[0051] 1) preparing materials, preparing materials according to the components and parts by weight of a terbinafine hydrochloride compound sustained-release capsule, wherein terbinafine hydrochloride is pulverized...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com