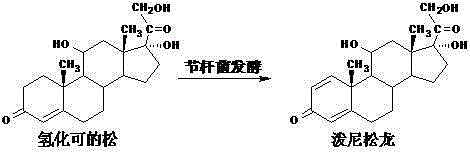
Preparation method of prednisolone
A technology of prednisolone and reaction, applied in the field of chemical pharmacy, can solve the problems of difficult separation and purification in the post-treatment process, low concentration of substrate feeding, long production cycle, etc., achieve novel process route, shorten production cycle, and low production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 750ml of acetone, 30g of prednisolone intermediate reduction, 12g of sodium acetate, 50ml of acetic anhydride, and 8ml of acetic acid into the reaction flask in sequence, and heat up and reflux for 4 hours. TLC showed that the reaction was complete. Most of the solvent was recovered by atmospheric distillation, and then evaporated to dryness under reduced pressure, cooled to below 5°C, stirred for 30 minutes, left to stand for more than 2 hours, filtered, and dried to obtain 32.0 g of esterified product.
[0046] Put 30g of the esterified product into a solution prepared by 150ml of 36% hydrochloric acid, 240ml of water, and 15ml of chloroform, and stir at 25°C to 30°C for 30 minutes to dissolve. Cool down, and add dropwise a solution prepared from 30 g of sodium nitrite and 1050 ml of water at 18°C to 22°C. After the addition was complete, the reaction was continued at 18°C to 22°C for 3 hours. TLC showed that the reaction was complete, lower the temperature t...
Embodiment 2
[0049] Add 750ml of acetone, 30g of prednisolone intermediate reduced product, 15g of potassium acetate, 51ml of acetic anhydride, and 9ml of acetic acid into the reaction flask in sequence, and heat up and reflux for 4.5 hours. TLC showed that the reaction was complete, most of the solvent was recovered by atmospheric distillation, and then the solvent was evaporated under reduced pressure, cooled to below 5°C, stirred for 30 minutes, left to stand for more than 2 hours, filtered, and dried to obtain 31.9g of esterified product.
[0050] Put 30g of the esterified product into a solution prepared by 150ml of 36% hydrochloric acid, 240ml of water, and 15ml of chloroform, and stir at 25°C to 30°C for 30 minutes to dissolve. Cool down, and add dropwise a solution prepared from 30 g of sodium nitrite and 1050 ml of water at 18°C to 22°C. After the addition was complete, the reaction was continued at 18°C to 22°C for 3 hours. TLC showed that the reaction was complete, lower th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com