Skin flap infection and avascular necrosis prevention and treatment transdermal preparation and preparation method thereof
A technology of transdermal preparation and skin flap infection, which is applied in the field of medicine, can solve the problems of limited skin penetration area and affect the penetration effect of drugs, and achieve the effect of no systemic effect, reduction of toxic and side effects, and improvement of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment one gel
[0033] Prepare the gel as follows:
[0034] 1. Preparation of sodium carboxymethyl cellulose gel matrix: Weigh 5.0 g of sodium carboxymethyl cellulose raw material, disperse it with 10.0 g of 95% ethanol, add 65.0 g of distilled water, stir evenly, seal it, place it overnight, and make it fully Swell to form a hydrogel matrix.
[0035] 2. Preparation of gel:
[0036] Main drug formula:
[0037] 1.00% AZM Gel Formula 100.0mg AZM
[0038] 0.50% AB gel formula 50.0mg AB
[0039] 0.16% LMWH-Na gel formula 16.0mg LMWH-Na
[0040] Transdermal formulation for prevention and treatment of flap infection ischemic necrosis 100.0mg AZM+50.0mg AB+16.0mg LMWH-Na
[0041]Accurately weigh the main ingredients according to the above formula, measure 300.0mg of azone and 200.0mg of propylene glycol, put them in a 25ml beaker, add absolute ethanol until 2g is dissolved, add them into 8g of gel matrix, stir well, and make 1.00% AZM gel respecti...
Embodiment 2
[0042] Example 2 Preparation of Infected Flap Model of Random Ischemia
[0043] Three days before the operation, the back of the mice was depilated with 8% sodium sulfide. After anesthesia by intraperitoneal injection of pentobarbital sodium (0.3ml / 100g body weight), the rat was fixed on the operating table in a prone position, and a random flap of 10.0mm×70.0mm was designed with a pedicle of 20.0mm from the tail end. Routine disinfection with povidone iodine, incision of the skin along the designed line, the meat membrane reaching the superficial layer of the back sarcolemma, sharp separation along this layer to the pedicle of the flap, and in situ intermittent sutures with 3 / 0 silk sutures after thorough hemostasis.
[0044] After the standard strains of Staphylococcus aureus and pathogenic Escherichia coli were revived in a 4°C incubator, two colonies were picked in a sterile operating table and placed in LB liquid medium, and slowly oscillated in a constant temperature sha...
Embodiment 3
[0046] Example 3 Detection of AZM and AB Contents in Rat Plasma and Flap Tissue
[0047] 1. Construction of AZM and AB content determination methods
[0048] (1) Chromatographic conditions Chromatographic column: Luna C 18 Column (250×4.60mm, 5μm); mobile phase: acetonitrile-phosphate buffer (0.02mol L -1 Potassium dihydrogen phosphate, use 1mol L -1 Sodium hydroxide to adjust to PH8.0) (55:45); flow rate: 1.0mL min -1 ; Column temperature: 30° C.; Detection wavelength: 210 nm; Injection volume: 20 μL.
[0049] (2) Preparation of reference substance solution Accurately weigh 30.0mg of AZM and 20.0mg of AB respectively, dissolve them in acetonitrile and dilute to volume in a 10.0mL volumetric flask to obtain a concentration of 2000μg·mL -1 standard solution, ready for use.
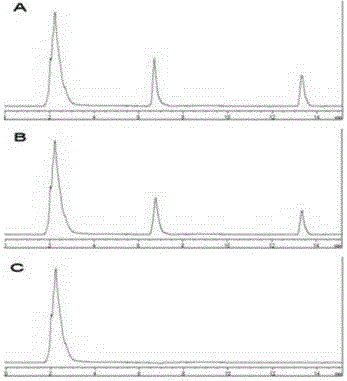
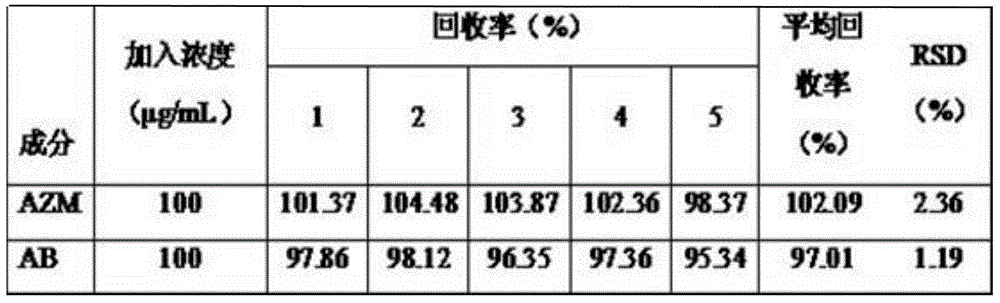
[0050] (3) System suitability test Under the specified chromatographic conditions, accurately draw the reference solution, the sample solution, and 20.0 μL of physiological saline, respectively, and in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com