Method for utilizing human butyrylcholinesterase minigene to express recombinant human butyrylcholinesterase in mammary gland
A human butyrylcholine and gene technology, applied in the field of genetic engineering, can solve the problems of difficult gene manipulation, low efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
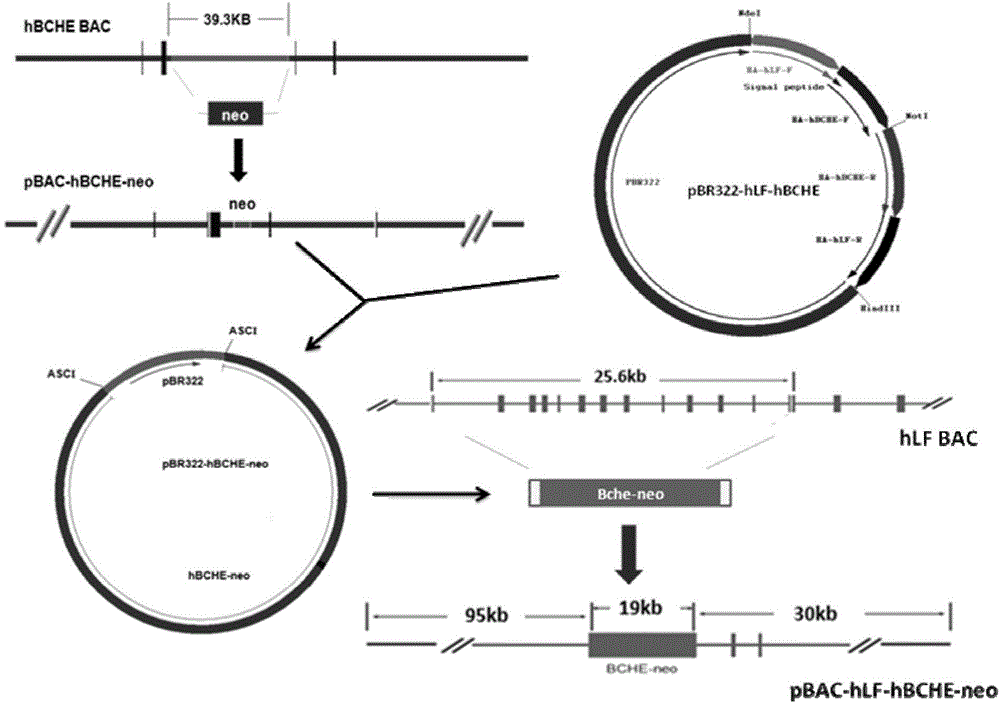
[0041]Example 1 Construction of mammary gland-specific expression of human butyrylcholinesterase vector pBAC-hLF-hBCHE-neo
[0042] 1. Construction strategy of human butyrylcholinesterase mammary gland-specific expression vector pBAC-hLF-hBCHE-neo
[0043] 1.1 Construction of capture vector pBR322-hLF-hBCHE
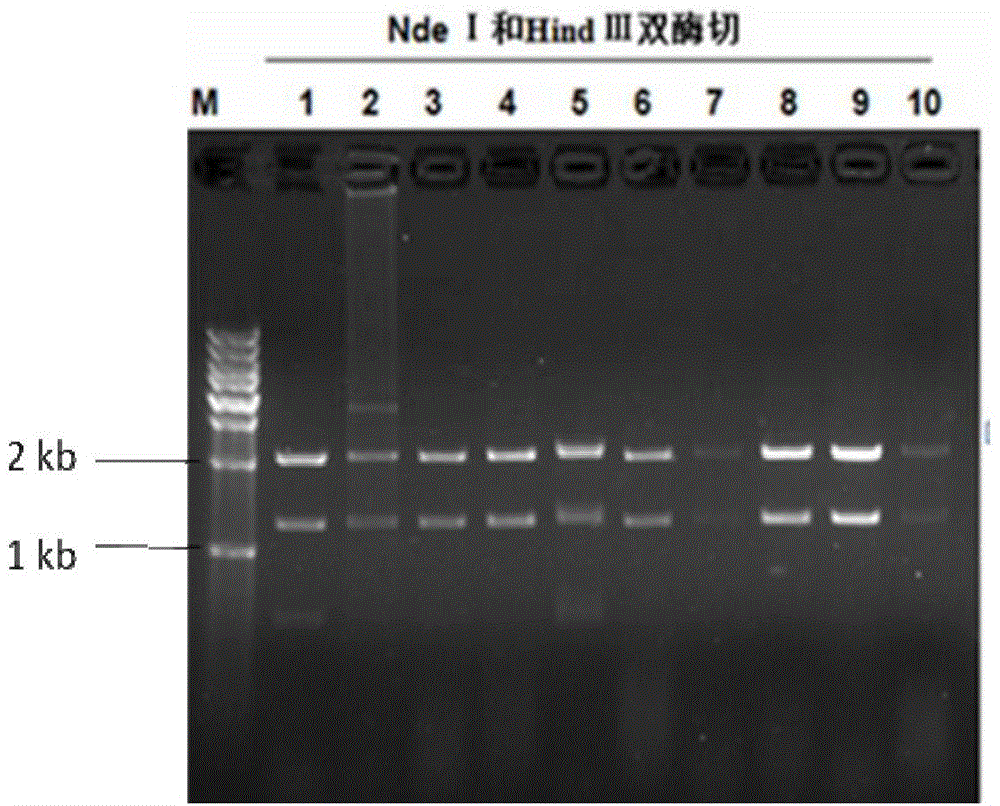
[0044] Using hLF BAC and hBCHE BAC as templates to amplify the homologous arms used in the homologous recombination operation, the PCR products of the 4 homologous arms were connected into the pMD19-T vector and sequenced to verify correctness. The four homology arms were connected into a fragment in the order of HA-hLF-F, HA-hBCHE-F, HA-hBCHE-R and HA-hLF-R by fusion PCR method, and connected to pBR322 by NdeI and HindIII double enzyme digestion Among the backbone vectors, the capture vector pBR322-hLF-hBCHE was obtained.
[0045] 1.2. Modification of human butyrylcholinesterase BAC carrier
[0046] The genomic DNA of human butyrylcholinesterase is about 80kb in lengt...
Embodiment 2
[0087] Example 2 Establishment and detection of human butyrylcholinesterase transgenic mouse model
[0088] 1. Production of Transgenic Mice by Microinjection
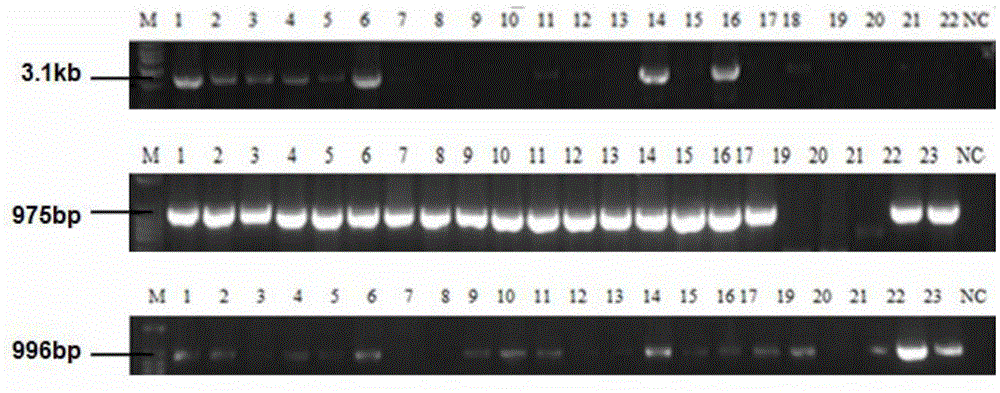
[0089] For the preparation process of transgenic mice, please refer to "Experimental Guidelines for Mouse Embryo Operation" (A. Nagy et al., 2004, Science Press). Transgenic efficiency. The production process of transgenic mice is as follows: Image 6 shown.
[0090] Microinjection is used to produce transgenic mice. For ordinary small fragment vectors, the linear integration efficiency is higher than that of circular ones. Therefore, the constructed expression vectors must be enzymatically cut into linear shapes for microinjection. However, due to its large structure, the large-fragment BAC vector is prone to breakage during the recovery process after linearization. If it is not recovered, the same high transgenic efficiency can be obtained, which will greatly increase the success rate and save working time. In ou...
Embodiment 3
[0099] Determination of recombinant human butyrylcholinesterase in the milk sample of embodiment 3 transgenic mice
[0100] 1. Western blotting detection of transgenic mouse milk samples
[0101] The normal milk of F0 generation 4, 5 and F1 generation 7, 9, 11 mice was collected, and the milk of negative mice was used as a negative control. The milk was collected one week after the birth of the mice, and the mother mice were separated from the pups for more than 3 hours before collection, and a certain dose of oxytocin was injected into the abdominal cavity. Whole milk and milk samples treated with defatted and decaseinized milk were electrophoresed on 10% SDS-PAGE gel. After the electrophoresis was completed, transfer to the membrane for 80 minutes using a Bio-Rad wet-transfer instrument at 350 mA. After the transfer was completed, block overnight with 5% skimmed milk powder, then incubate with rabbit anti-human primary antibody (diluted 1:500) for 1 h, wash the membrane wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com