Alligator mississrppinsis Cathelicidin-AM antibacterial peptide as well as coded sequence and application thereof
An antibacterial peptide and alligator technology, applied in the field of antibacterial peptides, can solve the problems of complex methods of antibacterial peptides, high cost of antibacterial peptides, and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment one: the solid-phase chemical synthesis method of Cathelicidin-AM antimicrobial peptide
[0076]The amino acid sequence of Cathelicidin-AM mature peptide was deduced according to the gene encoding American alligator Cathelicidin antimicrobial peptide, and the Cathelicidin-AM antimicrobial peptide was artificially synthesized according to the standard solid-phase peptide synthesis procedure. The synthesized peptide was desalted and purified by reverse phase-high pressure liquid chromatography (RP-HPLC, Column: X Bridge 4.6×250mm), and eluted with acetonitrile / water / trifluoroacetic acid system (Pump A: 0.07% trifluoroacetic in 100% water ; Pump B: 0.05% trifluoroacetic in 100% acetonitrile), and its molecular weight was determined by electrospray mass spectrometry (ESI-MS). The purity of the sample is ≥96%, the theoretical molecular weight is 4546, and the measured molecular weight is 4547.70, which are basically the same. The synthesized Cathelicidin-AM antib...
Embodiment 2
[0077] Example 2: Acquisition of American Alligator Cathelicidin-AM Antimicrobial Peptide cDNA
[0078] The inventors used TaKaRa Smart TM The cDNA library construction kit constructed the American alligator skin tissue cDNA library, sequenced randomly selected clones, and compared the measured sequence with the sequence in the international gene bank (GenBank) by BLAST to obtain the Cathelicidin-AM antibacterial Peptide cDNA.
[0079] 1. Extraction of total RNA from epithelial tissue of American alligator mucosa
[0080] The epithelial tissues of the American alligator mucosa were collected in cryopreservation tubes, and then the American alligators were released. The cryopreservation tubes were placed in liquid nitrogen and transported back to the laboratory, where they were stored in liquid nitrogen tanks for later use.
[0081] Take 0.1 g of mucosal epithelial tissue, grind it into powder at low temperature with liquid nitrogen. Add 2mL RNAiso Reagen and let it stand ...
Embodiment 3
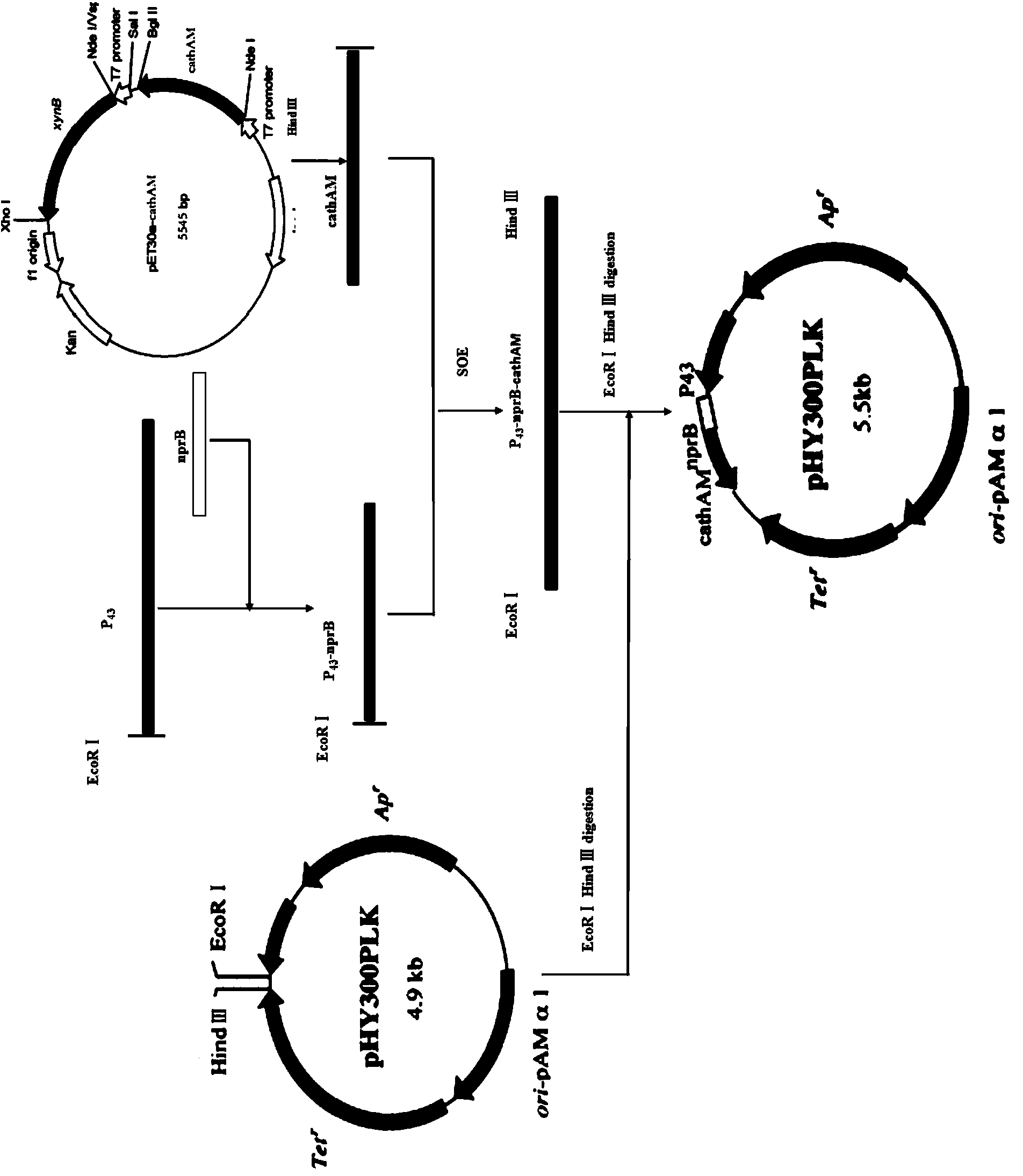
[0123] Example 3: Recombinant expression of American alligator Cathelicidin antimicrobial peptide in Bacillus subtilis
[0124] 1. According to the sequence at both ends of the 17-139 polynucleotide in SQ ID NO: 1, the primers are calculated as follows:
[0125] P1: 5'-GTGGGGTTCAAAGAAAGGCCTG-3';
[0126] P2: 5'-CCCAAGCTT CTAGAACTTGCCAAGGAACTTGGC-3' (introduced Hind III restriction site).
[0127] Using the pET30a-cathAM plasmid as a template to amplify the Cathelicidin-AM gene, the PCR system is as follows:
[0128]
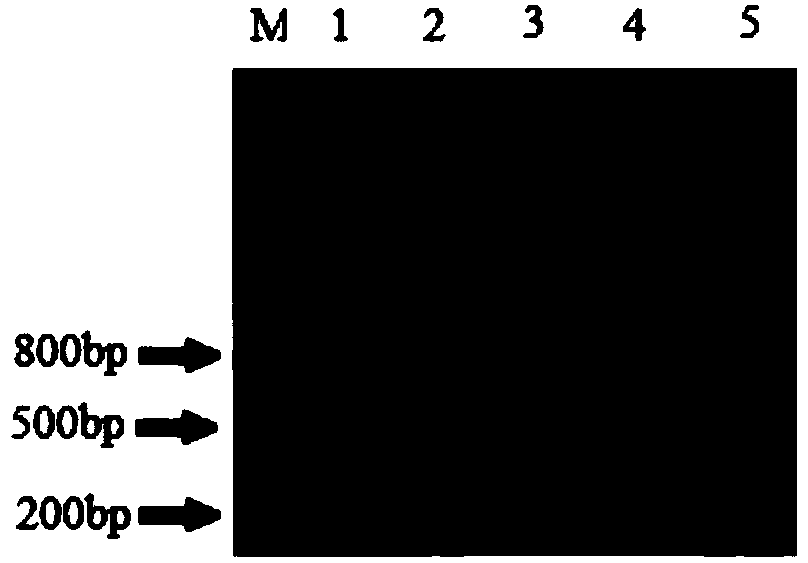
[0129] Reaction program: pre-denaturation at 94°C for 3min; denaturation at 94°C for 30s, annealing at 57°C for 30s, extension at 72°C for 1min, 35 cycles; extension at 72°C for 10min. 1% agarose gel electrophoresis to detect PCR products (see electrophoresis results image 3 ).
[0130] 2. Using the genomic DNA of Bacillus subtilis as a template, primers were set according to the sequence known in Genbank to amplify P 43 Promoter sequence, EcoR Ⅰ restr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com