Fluoro-carbostyril derivative as well as preparation method and application thereof
A fluoroquinolone and fluoroquinolone technology, which is applied in the field of novel fluoroquinolone derivatives and their preparation, can solve the problems of inconvenient industrial production, difficult post-processing, and high reaction temperature, and achieve low price of raw materials, convenient post-processing, and total yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
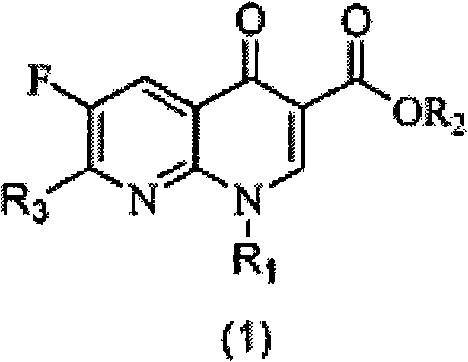
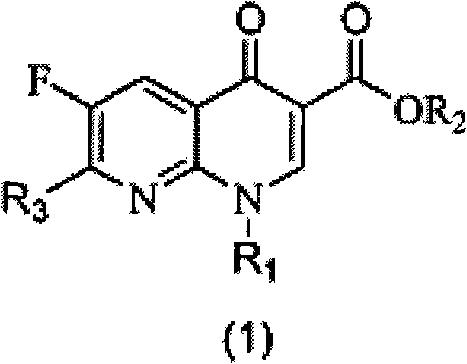
[0051] Example 1: Ethyl 2-(2,6-dichloro-3-fluoronicotinoyl)-3-naphthylaminoacrylate (S1)
[0052] Fluclonicotinate (4g, 14.3mmol) was dispersed in a mixed solution of acetic anhydride (10mL) and triethyl orthoformate (2.7mL, 16.3mmol) for reflux reaction for 1 hour, and excess acetic anhydride and orthoformic acid were removed under vacuum Triethyl ester, the resulting brown oil was diluted with 15 mL of absolute ethanol, then naphthylamine (16 mmol) was added and stirred at room temperature until a large amount of precipitation occurred. After filtering the resulting precipitate, the filter cake was washed with a small amount of methanol, and the filter cake was dried to obtain intermediate S1 (5.35 g). The two-step total yield was 86.3% (based on flunicotinate).
Embodiment 2
[0053] Example 2: Ethyl 7-chloro-6-fluoro-1-naphthyl-4-oxo-1,4-dihydro-1,8-naphthyridinyl-3-carboxylate (1)
[0054] Take S1 (500 mg) and disperse it in 2.5 mL of acetonitrile, then add 0.5 g of anhydrous potassium carbonate, and react under reflux until the reaction of raw materials is detected by thin layer chromatography. After cooling, the reaction system was poured into 50 mL of water and fully stirred for 30 minutes before filtration. The filter cake was washed with a small amount of methanol and dried to obtain Compound 1 (275 mg). The yield was 58.9%. 1 H NMR (300MHz, CDCl 3 ): δ8.63(s, 1H), 8.52(d, 1H), 8.09-7.99(dd, 2H), 7.66-7.24(m, 4H), 7.23(t, 1H), 4.40-4.33(m, 2H ), 1.36(t, 3H).
Embodiment 3
[0055] Example 3: Ethyl 2-(2,6-dichloro-3-fluoronicotinoyl)-3-phenylaminoacrylate (S2)
[0056] Aniline (16mmol) was added to react in the oily matter obtained in the first step, and the reaction operation was the same as in Example 1. After filtration and drying, the intermediate S 2 (4.07 g) was obtained, and the total yield of the two steps was 74.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com