Chiral-separation solid membrane grafted by chiral identification body through dopamine pretreatment, and making method thereof
A chiral recognition and chiral separation technology, applied in organic chemistry methods, chemical instruments and methods, membrane technology, etc. Effects of modification, expanded selectivity, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
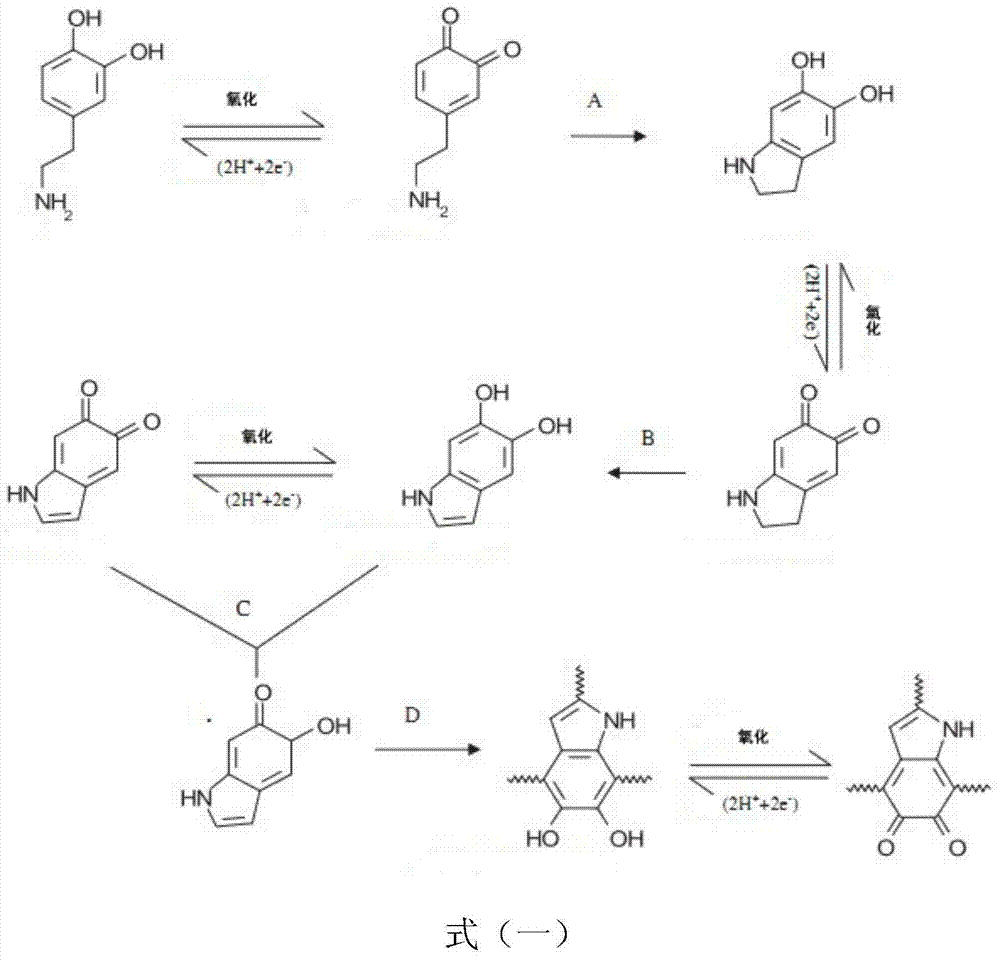
[0036] (1) β-CD-NH 2 Preparation: 12.0g β-CD was added to 100mL distilled water, 1.32g NaOH was dissolved in 4mL water, and added dropwise to the β-CD solution, the original suspension gradually became uniform. Then 2.24g of p-toluenesulfonyl chloride was dissolved in 8mL of acetonitrile, and added dropwise to the β-CD solution, a white precipitate appeared, reacted at 25°C for 2h, and placed at 4°C overnight. The white solid was obtained by filtration, soaked in diethyl ether, filtered and washed twice with hot water, and dried under vacuum to obtain the intermediate 6-OTs-β-CD. Take 1.0g of 6-OTs-β-CD and react with 6g of ethylenediamine, reflux at 70°C for 4h, the obtained solution is slightly diluted with a certain amount of methanol, and precipitated in acetonitrile solution to obtain a white solid, which is and dried overnight to obtain β-CD-NH 2 . Dissolve 1 g of dopamine (Aladdin Reagent Co., Ltd.) in 1 L of Tris-buffer (pH 8.0) to prepare 1 g / L of dopamine Tris-buf...
Embodiment 2
[0042] (1) Soak the flat polyvinylidene fluoride membrane (Guangzhou Chaoyu Membrane Separation Technology Co., Ltd.) in isopropanol (98%) for 90 minutes to completely wet the membrane.
[0043] (2) Take out the soaked polyvinylidene fluoride flat film and soak it in 2g / L dopamine Tris buffer solution (pH8.5), expose it to the air at room temperature, stir for 24 hours, take out the film and wash it with water after the reaction is completed net.
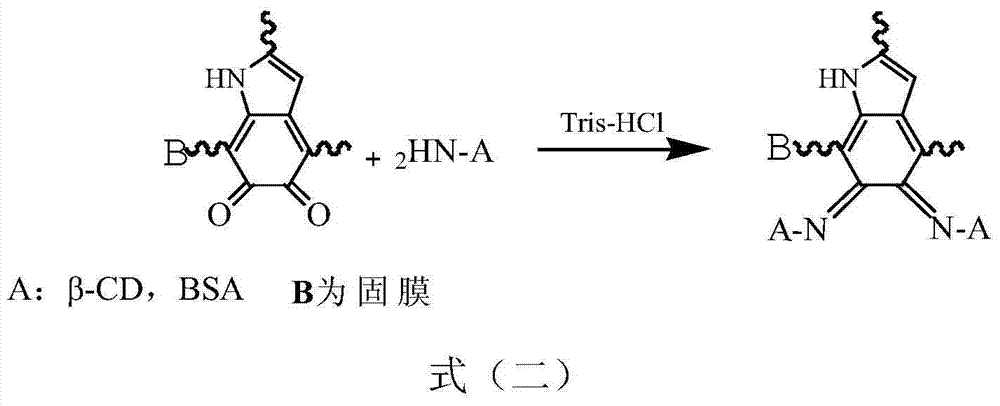
[0044] (3) Soak the dopamine-treated solid film in 2g / L BSA-NH 2 In Tris buffer (pH8.5), react at 20°C for 2h. After the reaction, the membrane was washed repeatedly with water and dried to obtain a chiral resolution solid membrane.
[0045] The flow rate of polyvinylidene fluoride flat membrane before modification is 90L m -2 h -1 , the flow rate of modified polyvinylidene fluoride plate chiral separation membrane is 245L m -2 h -1. The resolution efficiency (e.e%) for D,L-histidine was 29%, and the resolution efficiency (e....
Embodiment 3
[0047] (1) Soak the polytetrafluoroethylene tubular membrane (Guangzhou Ruite Water Purification Technology Co., Ltd.) in isobutanol (98%) for 240 minutes to completely wet the membrane. Take 5 g of dopamine (Aladdin Reagent Co., Ltd.) and dissolve it in 1 L of Tris-buffer (pH9.0) to prepare 5 g / L dopamine Tris-buffer. Take 1g β-CD-NH 2 Dissolve in 5L of Tris-buffer (pH9.0) to prepare 5g / L of β-CD-NH 2 Tris-buffer.
[0048] (2) Take out the soaked polytetrafluoroethylene tubular membrane and soak it in 5g / L dopamine Tris buffer solution (pH9.0), expose it to the air at room temperature, stir for 48h, take out the membrane and wash it with water after the reaction is completed net.
[0049] (3) Soak the dopamine-treated solid film in 5g / L β-CD-NH 2 In Tris buffer (pH9.0), react at 70°C for 4h. After the reaction, the membrane was washed repeatedly with water and dried to obtain a chiral resolution solid membrane.
[0050] The flow rate of PTFE tubular membrane before mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com