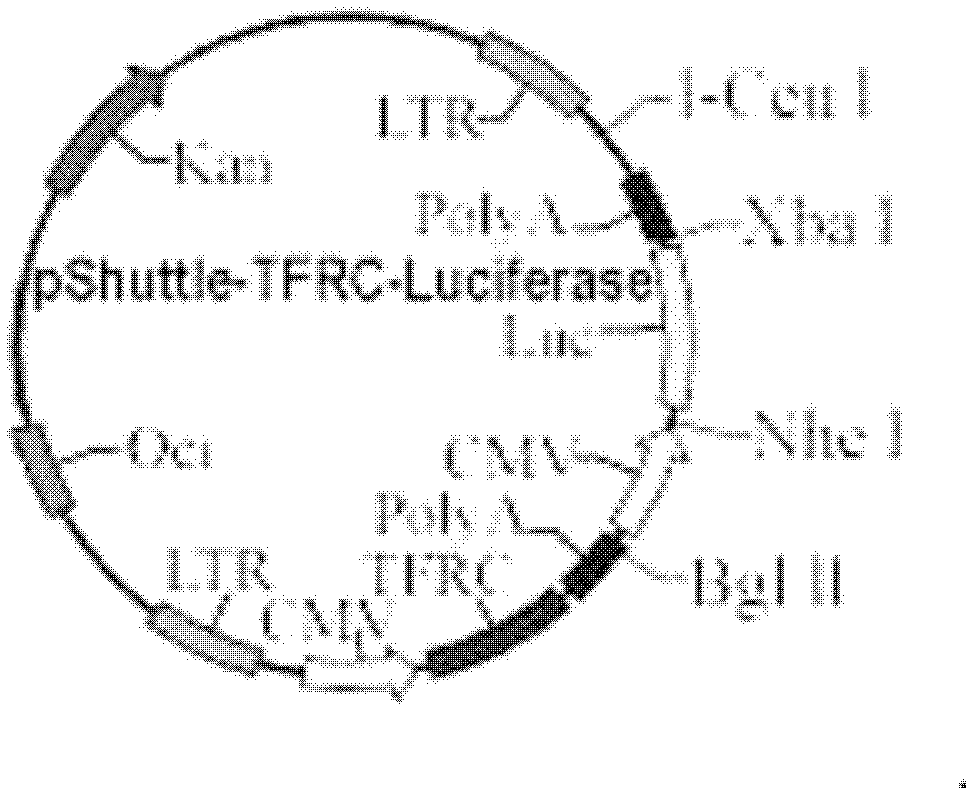Double-reporter-gene recombinant adenovirus vector, construction method and application thereof
A recombinant adenovirus and vector technology, applied in the field of genetic engineering, can solve the problem of difficult to meet the requirements of in vivo optical and magnetic resonance dual-modal imaging tracking at the same time, so as to increase biological safety, improve adaptability and stability, and achieve transformation. High dyeing efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction of recombinant adenoviral vector
[0033] 1. Materials and methods
[0034] 1.1 Target gene, vector plasmid, cell
[0035] The original cDNA plasmid of the human transferrin receptor gene (TFRC): GeneCopoeia, USA; the shuttle vector pShuttle-CMV-Luciferase and the chemically competent cell DH5α strain: Shanghai Hanheng Company; the backbone vector pAdenoviral Vector (pAdeno for short): Qbiogene, USA; human embryonic kidney cell line HEK293 cells: Cell Center, Institute of Basic Medical Sciences, Peking Union Medical College, China.
[0036] 1.2 Reagents
[0037] The restriction endonucleases used were all purchased from New England Biolabs; T4DNA ligase was MBI (Fermentas) product of Jingmei Biological Company; CIP enzyme (Alkaline Phosphatase, Calf Intestinal) was purchased from Promega; pfx DNA polymerase was purchased from Invitrogen; Small / medium-volume extraction and purification kits, DNA purification kits (column centrifugal type), DNA ...
Embodiment 2
[0077] Example 2: Infection and expression experiment of recombinant adenovirus vector Ad-CMV-TFRC-CMV-Luciferase on human colorectal cancer tumor cell LOVO
[0078] Human colorectal cancer tumor cell LOVO came from the Cell Center of Institute of Basic Medical Sciences, Peking Union Medical College, China, and the control Ad-GFP adenovirus came from Shanghai Hanheng Company (GFP stands for green fluorescent protein). Proceed as follows:
[0079] (1) Human colorectal cancer tumor cell LOVO was divided into 1×10 6 Inoculate each well into a 6-well plate, continue culturing for 24 hours, and replace the culture medium before infection;
[0080] (2) Add the recombinant adenovirus vector Ad-CMV-TFRC-CMV-Luciferase prepared in Example 1 and the control Ad-GFP adenovirus to infect human colorectal cancer tumor cell LOVO respectively, according to 1×10 10 / ml titer, that is, adding 5 μl of recombinant adenovirus vector Ad-CMV-TFRC-CMV-Luciferase or control Ad-GFP adenovirus solutio...
Embodiment 3
[0083] Example 3: In vivo tumor tracing experiment of recombinant adenovirus vector Ad-CMV-TFRC-CMV-Luciferase
[0084] 1.1 Instrument
[0085] In vivo fluorescence imaging system: IVIS, Caliper; 7.0T micro-MR imaging system: Bruker, Germany.
[0086] 1.2 Experimental animals
[0087] BALB / c nude mice, 4-5 weeks old, weighing 15-18g, 15 were purchased from Chengdu Dashuo Experimental Animal Technology Co., Ltd., and were raised in SPF (Specific Pathogen Free) environment breeding room in the Animal Experiment Center of Sichuan University. All experimental procedures were approved by the Management Committee for the Use of Experimental Animals of the Institute of Experimental Animals.
[0088] 1.3 Experimental method
[0089] The recombinant adenovirus vector Ad-CMV-TFRC-CMV-Luciferase successfully constructed in Example 1 was used to infect human colorectal cancer tumor cells LOVO at an optimal multiplicity of infection of MOI=50, and the recombinant adenovirus was inoculat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com