Aromatic diamine compound containing imide structure and its preparation method and application
An aromatic diamine and imide-containing technology, which is applied in the field of aromatic diamine compounds and their preparation, can solve the problems of imperfect mechanism and insufficient application, and achieve unique fluorescent characteristics, simple process, good thermal stability and solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
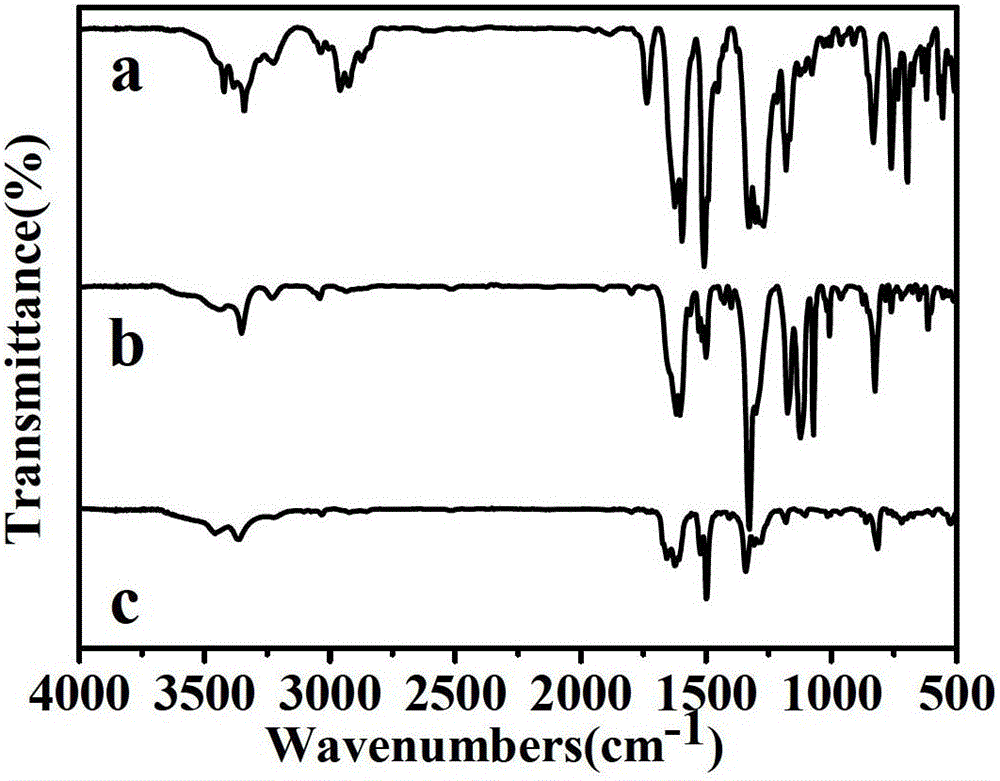
Image
Examples
Embodiment 1
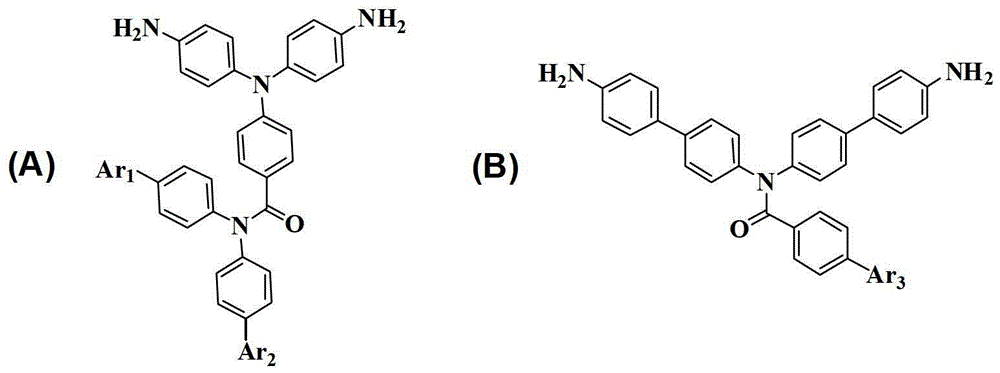
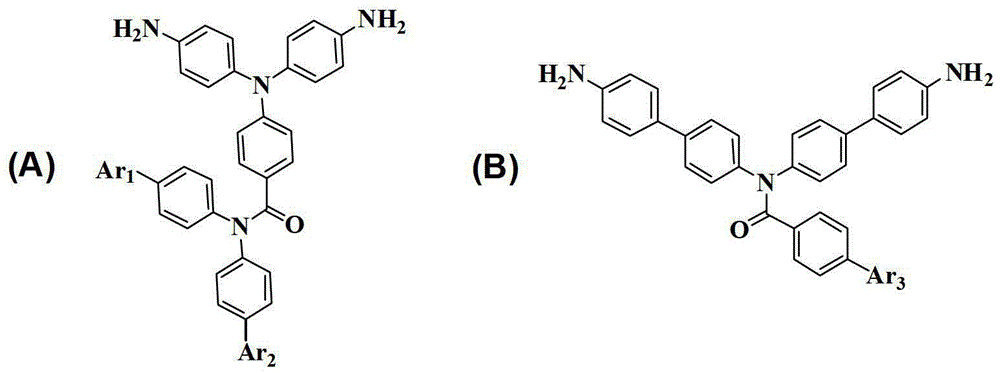
[0034] Example 1: Synthesis of 4-(Bis(4-aminophenyl)amino)-N,N-diphenylbenzamide, whose structural formula is as follows:
[0035] (1) Synthesis of intermediate 4-Nitro-N,N-diphenylbenzamide
[0036] Weigh 3.3846g (20mmol) of diphenylamine into a 100ml three-necked flask, add 40ml of tetrahydrofuran and 2.0123ml (25mmol) of pyridine and pass through argon, stir for 30min under ice-salt bath conditions, and weigh an equimolar amount of p-nitro Benzoyl chloride was added to the three-necked flask in batches, and the reaction was stirred until the temperature reached room temperature, and the temperature was raised to 60°C for more than 6 hours. After the reaction was completed, the solvent tetrahydrofuran was spin-dried with a rotary evaporator, dissolved in dichloromethane, purified by column chromatography, and dried under vacuum at 60°C overnight to obtain 3.8 g of a light green product with a yield of 61%. The chemical formula of this intermediate is C19H14O3N2, the molec...
Embodiment 2
[0046] Example 2: Synthesis of 4-(Bis(4-aminophenyl)amino)-N,N-bis(4'-(trifluoromethyl)-[1,1'-biphenyl]-4-yl)-benzamide, whose structural formula is as follows Shown:
[0047]
[0048] (1) Synthesis of intermediate 4,4'-BisbromophenylAmine
[0049] Weigh 5g (29.6mmol) of diphenylamine into a 250ml three-neck flask, use 50ml of dimethylformamide (DMF) as a solvent, stir magnetically and pass in argon, stir for 30min under ice-salt bath conditions, weigh 59.2mmol (10.53 g) N-bromosuccinimide (NBS) was dissolved in 50ml of DMF, added dropwise into a three-necked flask and completed dropwise within 30min, and reacted for 6h under ice-salt bath conditions. After the reaction was completed, the reaction solution was poured into ice-salt water and vigorously stirred to precipitate a gray solid, which was filtered with suction and washed three times with saturated sodium bicarbonate (NaHCO3) solution, and dried in vacuum at 60°C overnight to obtain 8.8 g of the product, with a yie...
Embodiment 3
[0065] Example 3: Synthesis of N,N-bis(4'-amino-[1,1'-biphenyl]-4-yl)-4-nitrobenzamide, whose structural formula is as follows:
[0066]
[0067] (1) Synthesis of intermediate 4,4'-BisbromophenylAmine
[0068]
[0069] (2) Synthesis of intermediate N,N-bis(4-bromophenyl)-4-nitrobenzamide
[0070]
[0071] The above two intermediates were synthesized according to the method of Example 2.
[0072](3) Synthesis of diamine monomer N,N-bis(4'-amino-[1,1'-biphenyl]-4-yl)-4-nitrobenzamide
[0073] Add 11.9g (25mmol) of 4,4'-BisbromophenylAmine and 7.53g (55mmol) of p-aminophenyl borate hydrochloride into a 250ml three-neck flask, add 100ml of tetrahydrofuran as a solvent, and then add 82.5ml of 2M potassium carbonate solution, Magnetic stirring and argon flow, after the oil bath temperature was heated to 70°C, a catalytic amount of tetrakistriphenylphosphine palladium was added, and after reflux reaction for 24 hours, the reaction solution was spin-dried and purified by co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com