Synthesis and application of coumarin type dye sensitizer
A technology of coumarins and compounds, applied in the field of synthesis of coumarin-type dye sensitizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] In an ice bath and dry conditions, the POCl 3 (2 mL) was added dropwise to DMF (2 mL), stirred for half an hour to obtain Vilsmier's reagent. Dissolve 7-methoxy-3-(2’-thienyl)-coumarin (5 mmol) in DMF (15 mL), add Vilsmier reagent, and react at 60 °C for 8 h. After cooling, the reaction solution was poured into ice water (30 mL), adjusted to pH = 7 with 10% NaOH aqueous solution, and a large amount of precipitate was precipitated. Filter with suction, wash with water (30 mL×5 ), and then with a large amount of ethanol, and dry to obtain a yellow-brown solid. IIb: Yield 84.9%. Melting point 164~165 oC.
[0038] Example 3: Synthesis of 2-cyano-3-(5-(7-N,N-dimethyl-2-carbonyl-2H-benzopyran-3-yl)thiophen-2-yl)acrylic acid IIIa
example 2
[0039] Dissolve II a (2 mmol) and cyanoacetic acid (4 mmol) in acetonitrile (30 mL), add piperidine (0.5 mL), and heat to reflux for 6 h. Cool, filter with suction, and wash with acetonitrile (20 mL×3). Dry, CHCl 3 Recrystallization gave a dark red solid. IIIa: Yield 87.3 %. Melting point 282~283 oC; 1 H NMR (500 MHz, DMSO) δ: 8.67(s, 1H, C H =CCN), 8.43(s, 1H, coumarin-4- H ), 7.98 (d, J=4.3 Hz, 1H, thiophene- H ), 7.86(d, J=4.2 Hz, 1H, thiophene- H ), 7.57(d, J=9.0 Hz, 1H, Ar H ), 6.83 (dd, J=9.0, 2.3 Hz, 1H, Ar H ), 6.65(d, J=2.23 Hz, 1H, Ar H), 3.50(q, J=7.0 Hz, 4H, C H 2 CH 3 ), 1.16 (t, J=7.0 Hz, 6H, CH 2 C H 3 ); HR-ESI-MS for C 21 h 17 N 2 o 4 S: Found: 393.0927 [M-H] - ; Calcd. 393.0909.
[0040] Example 4: Synthesis of 2-cyano-3-(5-(7-methoxy-2-carbonyl-2H-benzopyran-3-yl)thiophen-2-yl)acrylic acid IIIb
example 3
[0041] Dissolve II b (2 mmol) and cyanoacetic acid (4 mmol) in acetonitrile (30 mL), add piperidine (0.5 mL), and heat at reflux for 6 h. Cool, filter with suction, and wash with acetonitrile (20 mL×3). Dry, CHCl 3 Recrystallization gave a dark red solid. IIIb: Yield 75.6%. Melting point > 300 oC; 1 H NMR (500 MHz, DMSO): δ 8.83 (s, 1H, C H =CCN ), 8.45 (s, 1H, coumarin-4- H ), 8.01~7.97(m, 2H, thiophene- H ), 7.77 (d, J = 8.7 Hz, 1H, Ar H ), 7.15 (s, 1H, Ar H ), 7.07 (d, J = 8.7, 1H, Ar H ), 3.91 (s, 3H, OC H 3 ); HR-ESI-MS for C 18 h 10 NO 5 S: Found: 352.0293 [M-H] - ; Calcd. 352.0280.
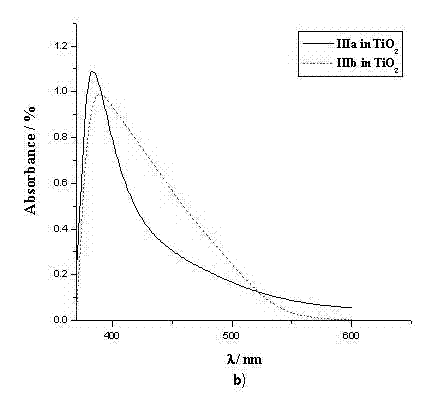
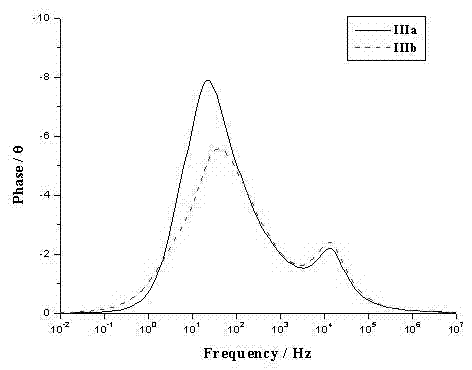
[0042] The spectral property test of example 5 dye sensitizers
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com