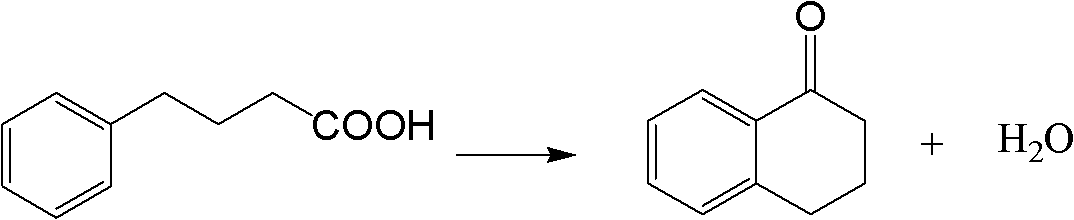
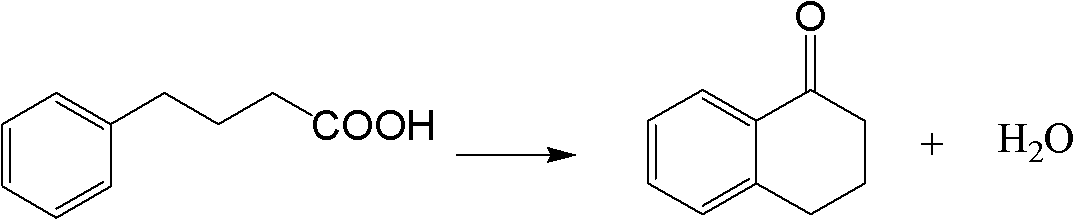
Method for synthesizing Alpha-tetralone through 4-phenylbutyric acid in catalytic way
A technology of phenylbutyric acid and tetralone, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problems of intermittent reaction, complex process, high cost, etc., and achieve the effect of improving production efficiency and optimizing conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
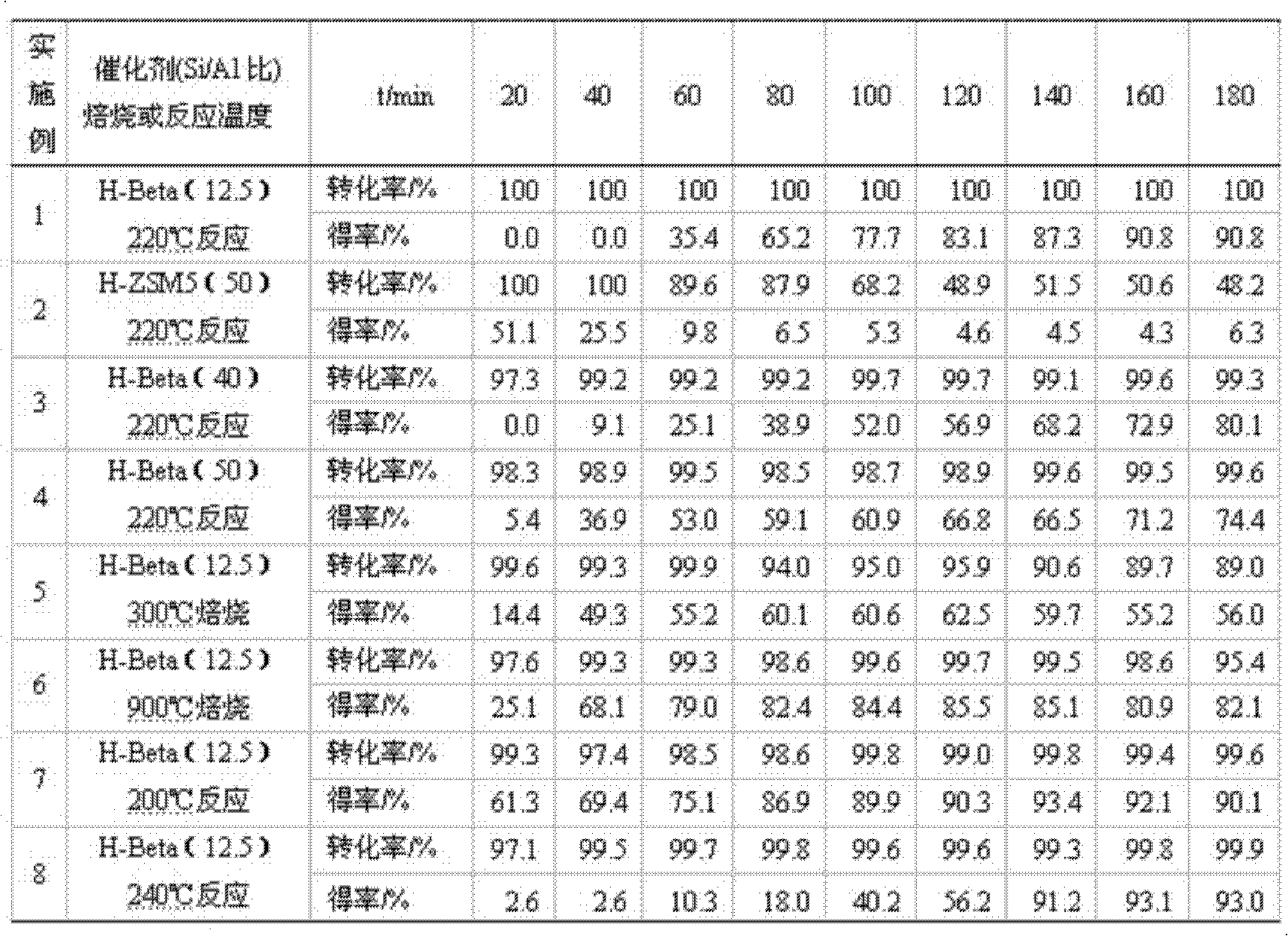
[0026] 1.0g of H-Beta catalyst (40-60 mesh, Si / Al=12.5, calcined at 500°C for 4h in an air atmosphere in a muffle furnace before use) was packed into a quartz tube with an inner diameter of 8mm and a length of 280mm, and connected In the reaction device, nitrogen gas (20mL / min) was introduced to activate at 500°C for 2h, and then cooled to 220°C. Use a constant flow pump to dissolve the mixed solution (4-phenylbutyric acid is dissolved in 1,2-dichlorobenzene according to the solid-to-liquid ratio of 1g / 10ml) at a certain injection rate (the liquid space velocity is 9.03h -1 ) into the top of the catalyst bed, the mixed liquid flows through the catalyst bed after being vaporized, and 4-phenylbutyric acid reacts on the catalyst; the product is condensed into liquid at the outlet of the reaction tube, collected once every 20min, The composition of the product was analyzed by GC-5190 gas chromatography, and the conversion rate of 4-phenylbutyric acid and the yield of α-tetralone w...
Embodiment 2
[0028] Under the same situation as other conditions and Example 1, the catalyst used was replaced by H-ZSM5 molecular sieve (Si / Al=50, 500 DEG C roasting 4h, 60-80 mesh), and its catalytic activity situation is listed in Table 1 (Example 2 )middle.
Embodiment 3~4
[0030] Under the identical situation of other conditions and embodiment 1, the catalyst used is replaced by the H-Beta molecular sieve (Si / Al=40,50) of different silicon-aluminum ratios, and its catalytic activity situation is listed in table 1 (embodiment 3~4) middle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com