Preparation process of dalteparin sodium
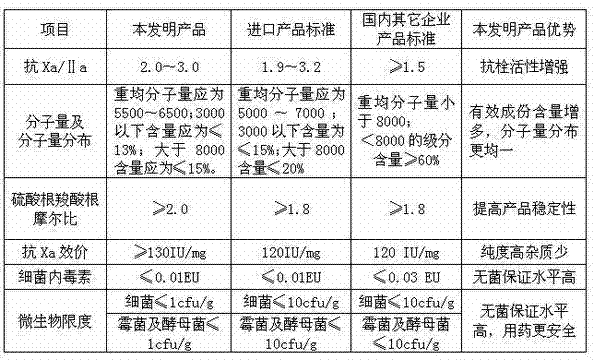
A technology for the preparation of dalteparin sodium, applied to medical preparations containing active ingredients, metabolic diseases, blood diseases, etc., can solve the problems of manganese dioxide difficult to filter out, quality control, and product recovery rate reduction, etc. Achieve the effects of easy control of the production process, prevention of unstable coronary heart disease, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
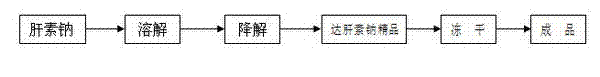
[0028] Embodiment 1: as figure 1 As shown, first:
[0029] ①. Weigh 3Kg sodium heparin, 45g sodium nitrite and 30g sodium borohydride respectively for later use;
[0030] ②. After adding 3Kg of heparin sodium into a 15-24L purified water reaction tank, stir at room temperature for 4-6 hours and dissolve to obtain a heparin sodium solution;
[0031] ③. After adjusting the pH of the above sodium heparin solution to 2~3 with hydrochloric acid, add 45g of sodium nitrite under stirring, then keep warm at 10~30°C and react for 2~5h under stirring, then let stand for 20 After ~24h, the heparin degradation solution was obtained;
[0032] ④. After adjusting the pH value of the above-mentioned heparin degradation solution to 9-11 with sodium hydroxide solution, add 30 g of sodium borohydride for reduction for 10-16 hours. After the reaction is completed, adjust the pH value to 6.5-7.0 with sodium hydroxide solution to obtain the reduction solution ;
[0033] ⑤. Add 2 to 4 times the ...
Embodiment 2
[0037] Example 2: The difference between this example and Example 1 is that the heparin sodium is 10Kg, the sodium nitrite is 150g, and the sodium borohydride is 100g. After freeze-drying, 9Kg of the finished product of dalteparin sodium is obtained.
Embodiment 3
[0038] Example 3: The difference between this example and Example 1 is that the heparin sodium is 30Kg, the sodium nitrite is 450g, and the sodium borohydride is 300g, and 27Kg of the finished product of dalteparin sodium is obtained after freeze-drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com