Medical degradable and absorbable Mg-Sr-Ca series magnesium alloy implant and preparation method thereof
A mg-sr-ca and implant technology are applied in the field of medical degradable and absorbable Mg-Sr-Ca magnesium alloy implants and their preparation to achieve optimal mechanical properties and corrosion resistance, inhibit bone resorption, promote The effect of tissue healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
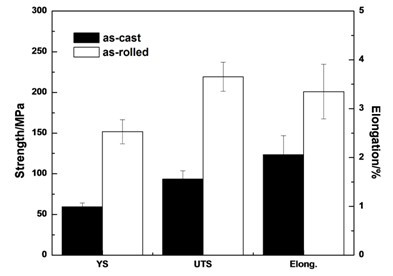
[0051] The test raw materials are pure Mg (99.95wt.%), Mg-Sr master alloy and Mg-Ca master alloy, according to the composition of Mg-Sr-Ca alloy (nominal composition by weight percentage: Sr 2%, Ca 1%, balance Alloyed for Mg), in CO 2 +SF 6 Melt the pure magnesium ingot under the protection of the atmosphere, raise the temperature to 750 ° C, add the Mg-Sr master alloy, stir for 10 minutes after it melts, then add the Mg-Ca master alloy, and stir for 10 minutes to homogenize the alloying elements Sr and Ca. After raising the temperature to 750°C and keeping it warm for 30 minutes, the melt was poured into a mold preheated to 300°C for gravity casting to obtain a Mg-Sr-Ca ingot. Adopt the as-cast Mg-Sr-Ca alloy microstructure that the present invention obtains as figure 1 shown. The as-cast Mg-Sr-Ca alloy was processed into a tensile standard sample, and the tensile test at room temperature was carried out at a tensile speed of 1 mm / min. The room temperature tensile propert...
Embodiment 2
[0053] The alloy smelting process is the same as in Example 1. The Mg-Sr-Ca alloy ingot is processed into a 6mm thick plate, sanded until there are no obvious defects, and kept at 400°C for 3 hours before rolling. The pass is returned to the furnace for 10 minutes, and finally rolled to a 1.2mm thick sheet. The as-rolled Mg-Sr-Ca alloy is processed into a tensile standard sample, and the tensile test at room temperature is carried out at a tensile speed of 1mm / min. The tensile properties at room temperature of the rolled Mg-Sr-Ca alloy obtained by the present invention are as follows: figure 2 shown.
Embodiment 3
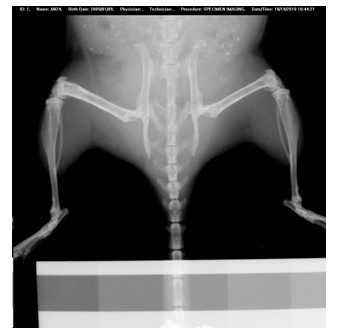
[0055] Process the obtained rolled Mg-Sr-Ca alloy into Φ0.7mm, 5mm long rods, grind and polish to 2000# sandpaper, and sterilize with ethylene oxide. Eight 3-month-old C57BL / 6 mice were selected and randomly divided into two groups with 4 mice in each group. In one group, the left knee was anesthetized and the skin was prepared and disinfected. A 0.7mm diameter hole was drilled in the distal femur, and an alloy rod was implanted in the hole and sutured. Another group of mice was drilled in the left femur as a blank control. Periodic observation after the operation did not reveal foreign body reactions such as inflammation of the surrounding tissue caused by the implant. Osteogenic response was found to be obvious 4 weeks after operation, the density of trabecular bone was higher than that of the control group, and the bone formation of the outer periosteum and inner periosteum were higher than those of the control group. Adopt the rolling state Mg-Sr-Ca alloy that the presen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com