Long-acting naltrexone implant and preparation method thereof
A technology of naltrexone and implants, which is applied in the field of long-acting naltrexone implants and its preparation, can solve the problems of inability to carry out large-scale production, different solution components, etc., and achieve the improvement of clinical treatment effect and release completion Good effect with few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
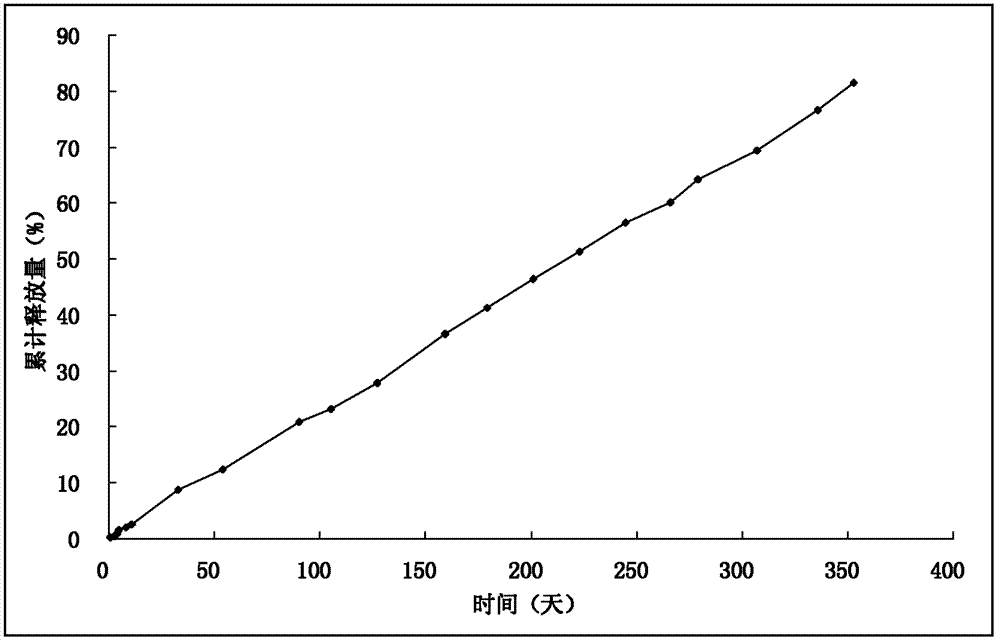
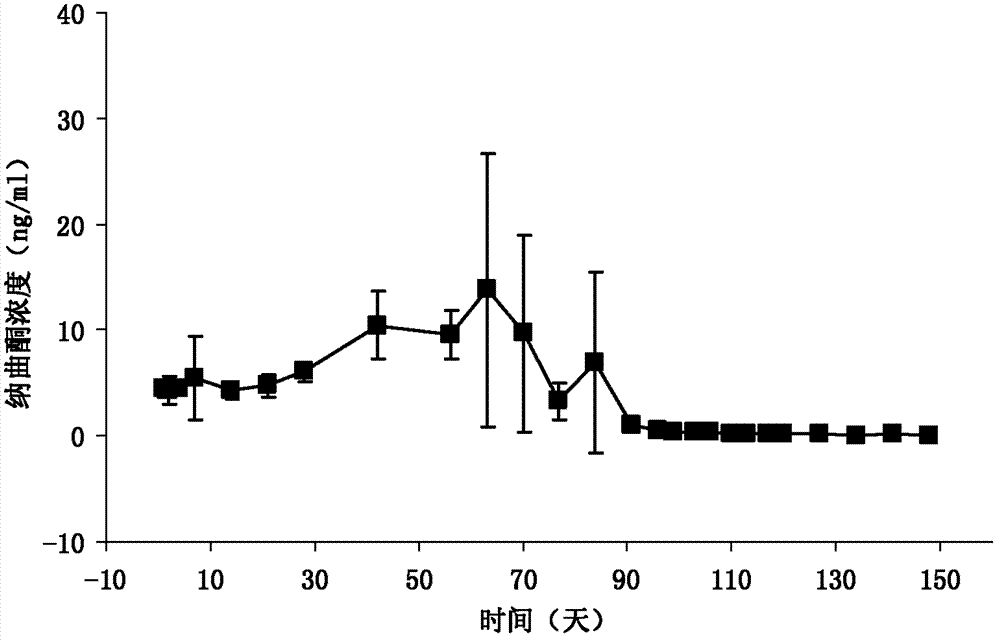
Image
Examples
Embodiment 1
[0033] DL-polylactic acid, the selected viscosity coefficient is 0.9dl / g, and the average molecular weight is about 50,000 Daltons. Dissolve DL-polylactic acid (20g) and naltrexone (20g) in dichloromethane (200mL) as the DP phase; prepare 6000ml of a 0.5% polyvinyl alcohol solution and saturate it with 10% sucrose as the CP phase ;The speed of the peristaltic pump is set to 180rpm, the nitrogen pressure is set to 2Kpa, and the stirrer speed is set to 180rpm; the DP phase is pressed into the CP phase from the outside through nitrogen, heated to 37°C, decompressed to a vacuum of -0.08Mpa, and continuously Stir; after the microspheres are solidified, cool to below 10°C in an ice-water bath, filter, wash the microspheres with distilled water 3 times, and vacuum-dry for 48 hours; combine the microspheres, pass through a 40-mesh sieve, mix well, and press into tablets with an appropriate pressure , the diameter of the tablet is 6.8mm; the surface is coated with DL-polylactic acid, e...
Embodiment 2
[0035] DL-polylactic acid, select viscosity coefficient 1.5dl / g (chloroform, 30°C), dissolve DL-polylactic acid (20g) and naltrexone (30g) in dichloromethane (200mL) as DP phase; prepare concentration It is 6000ml of 1% polyvinyl alcohol solution and saturated with 10% sucrose as the CP phase; the speed of the peristaltic pump is set to 200rpm, the nitrogen pressure is set to 8Kpa, and the stirrer speed is set to 180rpm; the DP phase is pressed into the CP from the outside by nitrogen phase, heated to 37°C, decompressed to a vacuum of -0.08Mpa, and continued to stir; after the microspheres solidified, cooled to below 10°C in an ice-water bath, filtered, washed the microspheres with distilled water 3 times, and dried in vacuum for 48 hours Merge the microspheres, pass through a 30-mesh sieve, mix well, and press into tablets with an appropriate pressure. Denaltrexone implant.
Embodiment 3
[0037] DL-polylactic acid, select viscosity coefficient 0.7dl / g, dissolve DL-polylactic acid (25g) and naltrexone (20g) in dichloromethane (250mL) as DP phase; prepare polyethylene with a concentration of 0.8% 6000ml of alcohol solution was saturated with 10% sucrose as CP phase; the speed of peristaltic pump was set to 200rpm, the pressure of nitrogen gas was set to 15Kpa, and the speed of stirrer was set to 180rpm; the DP phase was pressed into the CP phase from the outside by nitrogen gas, and heated to 37 ℃, reduce the pressure to a vacuum degree of -0.08Mpa, and continue to stir; after the microspheres are solidified, cool them in an ice-water bath to below 10°C, filter, wash the microspheres with distilled water three times, and dry them in vacuum for 48 hours; combine the microspheres, pass 60-mesh sieve, mixed, and pressed with appropriate pressure, the tablet diameter is 5.5mm; the surface is coated with DL-polylactic acid, the weight of each tablet is 280mg, the hardn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com