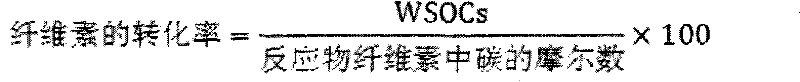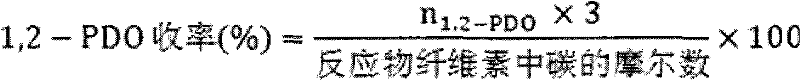Method for directly preparing glycols from biological cellulosan
A bio-fiber and polysaccharide technology, applied in the chemical industry, can solve the problems of low diol selectivity and achieve the effects of high yield, improved stability and less investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of Cu-ZnO, Ni-Cu-ZnO, Ni-ZnO catalysts:
[0028] A series of Ni-Cu-Zn catalysts with different metal ratios were prepared by co-current precipitation method. The preparation process is:
[0029] ① Make the precursor metal salt of the catalyst into a 1mol / L solution (change the ratio of the metal salt, but keep the total concentration of M2+ at 1mol / L);
[0030] ②Under full stirring, add the metal salt solution and 1.2mol / L sodium carbonate solution dropwise into 400mL deionized water preheated to 70°C at the same time, adjust the dropping speed so that the pH is about 8.0, and keep Keep the temperature constant, pH=8.0, continue to stir for 3h;
[0031] ③Washing: Let the formed emulsion stand still, cool to room temperature, add 800mL 50°C deionized water, stir evenly, let stand, pour out the supernatant, and then fully wash with suction;
[0032] ④ Drying and roasting: drying in a blast drying oven at 120°C for 12 hours, and roasting at 350°C for 4 hours...
Embodiment 2
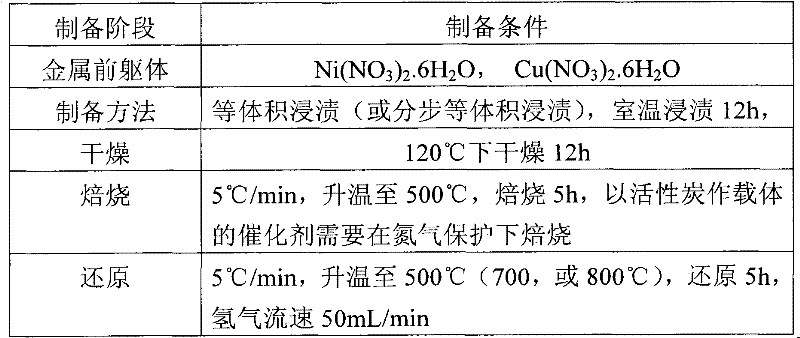
[0036] ZrO with high surface area 2 (Precipitation-hydrothermal method preparation, BET specific surface area up to 223m 2 / g) and commercial catalyst supports, such as activated carbon, microsphere silica gel, titanium dioxide, diatomaceous earth, alumina, etc. to prepare supported Ni-based, Ni-Cu-based catalysts. Activated carbon, microsphere silica gel, white carbon black, ZnO and other carriers are pretreated by drying, roasting, etc., and then measure their volumetric water absorption, and then prepare supported catalysts by equal volume impregnation method and stepwise equal volume impregnation method. The metal loading is 2wt%-20wt%, and the catalyst is expressed as xNiyCu / carrier, where x and y represent the loadings of the two metals respectively.
[0037] Table 1: Conditions for preparation of supported Ni and Ni-Cu catalysts by impregnation method
[0038]
Embodiment 3
[0040] Cellulose conversion experiment: the evaluation of cellulose hydrolysis-hydrogenolysis reaction is carried out in a high-pressure reactor, intermittent reaction, using green and environmentally friendly high-temperature and high-pressure water as the reaction medium, the catalyst is evaluated under milder conditions, and it is connected to a computer for recording Reaction temperature and pressure changes. Add 0.5g of cellulose, 0.15g of catalyst and 50mL of water into a 100m stainless steel autoclave, replace the gas with hydrogen for three times, fill with hydrogen to 4MPa, stir at a speed of 500 rpm, and heat up to 245°C for 0.5 ~8h. After the reaction, cool down to room temperature, take the centrifuged supernatant, pass through a 0.22 μm filter membrane, and separate and detect on gas chromatography (GC) and high performance ion chromatography (HPAEC-PAD).
[0041] Qualitative analysis of low-boiling point products by gas chromatography-mass spectrometry (GC-MS) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com