Polyethylene glycol-integrated interferon variant lyophilized preparation
A freeze-dried preparation, polyethylene glycol technology, applied in freeze-dried delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. Problems such as the high price of blood albumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of mPEG 20kDa -IFN-SA
[0038] IFN-SA solution (3.5mg / ml) is dissolved in sodium phosphate with a concentration of 100mM, pH6.0 contains 20mM NaCNBH 3 After cooling at 4°C, mix thoroughly, and add methoxypolyethylene glycol aldehyde (mPEG-propionaldehyde, average molecular weight 20kDa) in excess of 4 times the molar amount.
[0039] During the reaction, the modification degree of the protein was monitored by reversed-phase HPLC. After 10 hours, the reversed-phase HPLC analysis showed that 80% of the proteins with unblocked α-amino groups at the N-terminal were converted into mPEG-IFN-SA derivatives.
[0040] At the 10-hour time point, the reaction mixture was diluted 5-fold with water, and the purification of the mPEG-IFN-SA derivative was accomplished by ion-exchange chromatography on a Hiload 16 / 10 S Sepharose HP column buffered with 20 mM sodium acetate. The pH of the solution was balanced at 5.5, the reaction mixture was loaded into the colu...
Embodiment 2
[0041] Example 2 Prepare the freeze-dried stock solution made in the following proportions, and freeze-dry it into a finished product
[0042] Na 2 HPO 4 .12H 2 O, 2.58mg / ml, NaH 2 PO 4 .2H 2 O, 0.44mg / ml;
[0043] (1) 40mg / ml mannitol + 10mg / ml lysine hydrochloride;
[0044] (2) 35mg / ml mannitol + 12mg / ml arginine hydrochloride;
[0045] (3) 30mg / ml mannitol + 15mg / ml lysine hydrochloride;
[0046] (4) Mannitol of 40mg / ml;
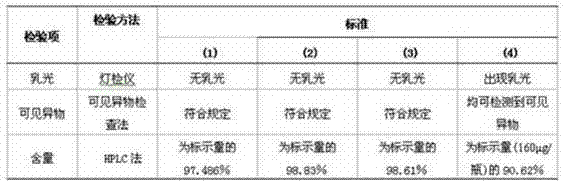
[0047] Proportionally, take mannitol, or different proportions of mannitol-lysine hydrochloride, or mannitol-arginine hydrochloride, prepare it with a phosphate buffer solution with a pH of 6.5-7.5, filter and sterilize, and prepare different proportions of mPEG20 of Mannitol or Dexmannitol-Amino Acid Cryoprotectant kDa - IFN-SA solution containing mPEG per ml of solution 20kDa -IFN-SA 50-160μg / ml, mix well, aliquot into 1ml / bottle, and freeze-dry at -40°C for 4 hours, then vacuum-dry at -40°C to -7°C After 20 hours, the temperature was gradua...
Embodiment 3
[0052] Example 3 mPEG 20kDa - IFN-SA lyophilized preparation
[0053] mPEG 20kDa -IFN-SA 160 μg / ml
[0054] Na 2 HPO 4 .12H 2 O 2.58mg / ml
[0055] NaH 2 PO 4 .2H 2 O 0.44mg / ml
[0056] Mannitol 38.6mg / ml
[0057] Lysine Hydrochloride 10.2mg / ml
[0058] Preparation method and process:
[0059] Accurately prepare the diluent according to the following diluent formula, and use it to dilute the original solution after preparation. The utensils used must be treated without pyrogens, and the whole process is operated under sterile conditions. Diluent formula: mannitol 38.6g, lysine hydrochloride 10.2g, Na 2 HPO 4 12H 2 O 2.58 g, NaH 2 PO 4 2H 2 O 0.44 g, the volume for injection is determined to be 1 L, and the pH of the diluent is about 7.2
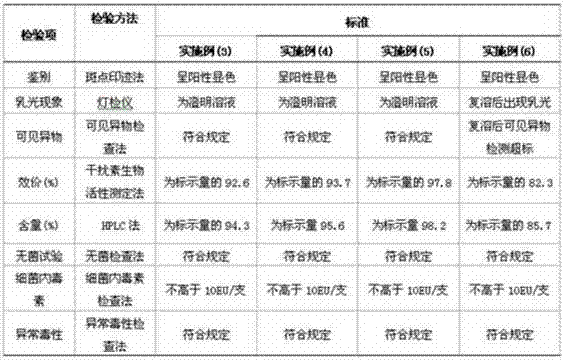
[0060] Take the qualified polyethylene glycol recombinant integrated interferon variant stock solution, accurately measure its volume, dilute with diluent to a protein concentration of 0.16 mg / ml, and filter aseptically to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com