Pulmonary delivery ciprofloxacin pharmaceutical composition and preparation method thereof
A technology of ciprofloxacin and pulmonary drug delivery, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve liposome leakage, affect the quality of preparations, lipid Insufficient stability of plastids and other problems, to achieve the effect of prolonging the action time, good stability, and easy to carry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
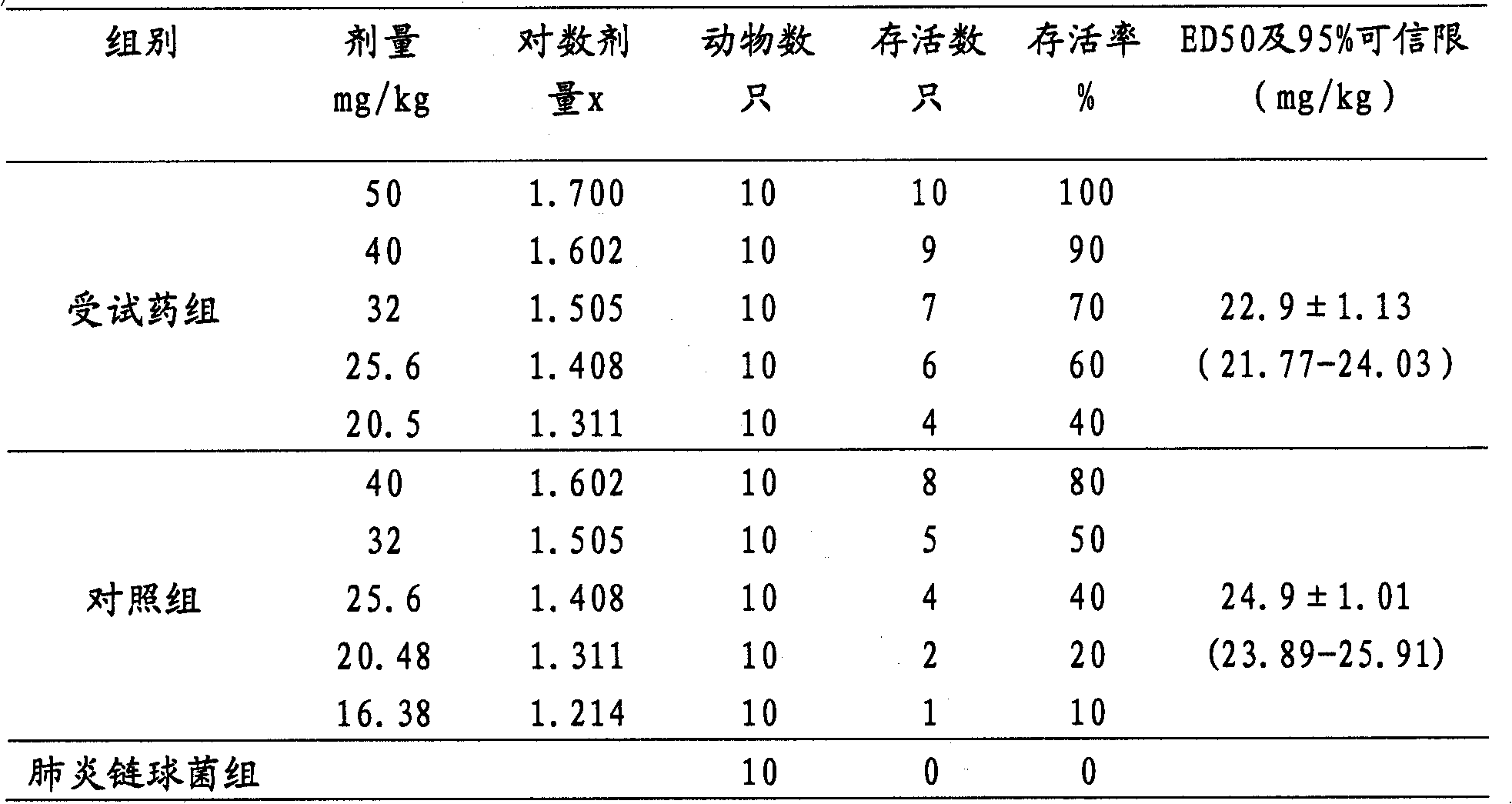
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of control sample
[0022] Prepare control samples with reference to the following literature:
[0023] Tejas R. Desai et al.2001. Determination of surface freeenergy of interactive dry powder liposome formulations using capillary penetration technique. Colloids and Surfaces B: Biointerfaces.22, 107-113; W.H.Finlay, J.P.Wong, 1998, Regional iposome lung deposition of neopos -encapsulated profloxacin. International Journal of Pharmaceuticals. 167, 121-127.
[0024] Specifically, the prescription is as follows:
[0025] Ciprofloxacin Hydrochloride 0.11g
[0026] DPPC 0.55g
[0027] Cholesterol 0.45g
[0028] Lactose 2.2g
[0029] 0.6M ammonium sulfate aqueous solution 20ml
[0030] Preparation method: Dissolve the prescribed amount of DPPC and cholesterol in chloroform and evaporate under reduced pressure, hydrate with 0.6M ammonium sulfate, ultrasonically granulate, dialyze with a certain amount of normal saline for 12 hours, add the p...
Embodiment 2
[0031] Embodiment 2: the preparation of spray formulation sample of the present invention
[0032] The prescription is as follows:
[0033] Ciprofloxacin Hydrochloride 0.11g
[0034] DPPC-PEG2000 0.55g
[0035] Cholesterol 0.45g
[0036] Tween 800.1ml
[0037] 0.6M ammonium sulfate aqueous solution 20ml
[0038] Preparation method: Take the prescribed amount of DPPC-PEG2000 and cholesterol, dissolve in chloroform and evaporate under reduced pressure, hydrate with 0.6M ammonium sulfate, ultrasonically granulate, dialyze with a certain amount of normal saline for 12 hours, add the prescribed amount of drugs, and incubate in a water bath at 50°C for 5 minutes . Mix this sample with Tween 80 and put it into a specific spray bottle to get sample 2.
Embodiment 3
[0039] Embodiment 3: the preparation of aerosol formulation sample of the present invention
[0040] The prescription is as follows:
[0041] Ciprofloxacin Hydrochloride 0.11g
[0042] DPPC-PEG2000 0.55g
[0043] Cholesterol 0.45g
[0044] Tween 80 0.1ml
[0045] Tetrafluoroethane (HFA134a) 3ml
[0046] 0.6M ammonium sulfate aqueous solution 20ml
[0047] Preparation method: Dissolve the prescribed amount of DPPC-PEG2000 and cholesterol in chloroform and evaporate under reduced pressure, hydrate with 0.6M ammonium sulfate, ultrasonically granulate, dialyze with a certain amount of normal saline for 12 hours, add the prescribed amount of drugs, and incubate in a water bath at 50°C 5min. Mix this sample with Tween 80 and put it into a specific container, then fill it with tetrafluoromethane to get sample 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com