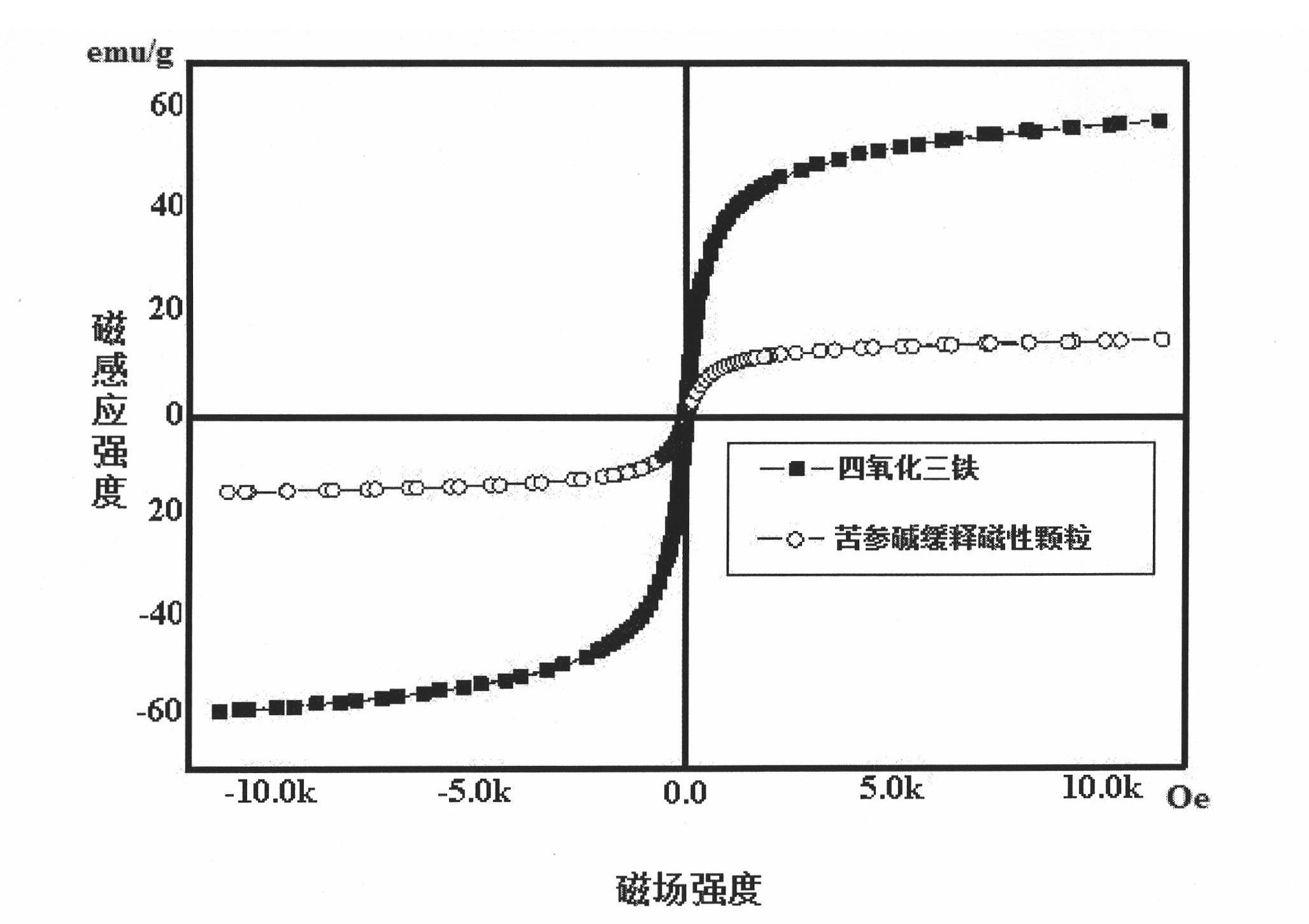
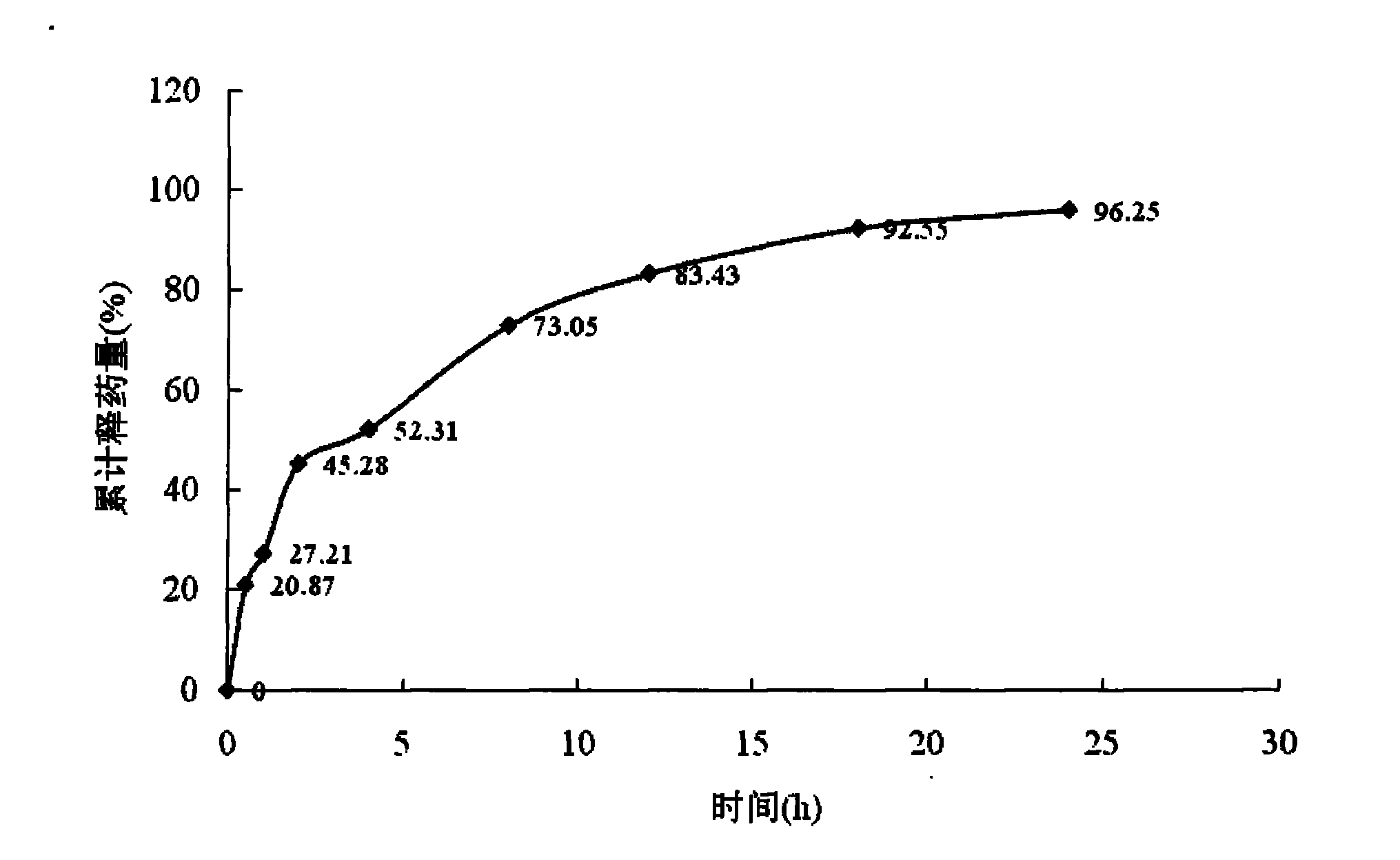
Matrine magnetic slow-releasing capsule and preparation method
A technology of sustained-release capsules and matrine, applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations of non-effective ingredients, etc., can solve the problem of ineffective reduction of drug side effects and adverse drug reactions, drug side effects and adverse effects Increased response, lack of targeted drug delivery characteristics, etc., to achieve improved bioavailability, high saturation magnetization, and sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) dissolving chitosan in an aqueous acetic acid solution with a volume ratio of 0.2% to 1%, and the concentration of chitosan in the aqueous acetic acid solution is 0.005 to 0.04g / mL to obtain an acetic acid solution of chitosan. The preparation method of chitosan in other embodiments of the present invention is the same as this.
[0031] (2) Preparation of matrine drug-loaded magnetic particles: Weigh microcrystalline cellulose (the cellulose content is 97.0% to 102%, and the colloid viscosity can reach 3000 to 5000mPa·s when the concentration is 3%, used in the following examples Each of the microcrystalline cellulose is the same) 0.4g, Fe 3 o 4 Magnetic nanoparticles (20nm, the magnetic nanoparticles particle size used in the following examples are the same) 0.12g and matrine 0.1g, after mixing, add 0.02g / mL chitosan in 1% acetic acid solution 2mL, stir well , Soft materials. Pass through a 65-mesh sieve to granulate, dry at a constant temperature of 50°C, sieve...
Embodiment 2
[0035] (1) Preparation of matrine drug-loaded magnetic particles: weigh 0.4 g of microcrystalline cellulose, Fe 3 o 4 After mixing 0.1 g of magnetic nanoparticles and 0.1 g of matrine, add 0.02 g / mL chitosan into 3 mL of 1% acetic acid solution, stir evenly, and make a soft material. Pass through a 65-mesh sieve to granulate, dry at a constant temperature of 50°C, sieve through a 65-mesh sieve, and granulate to obtain matrine drug-loaded magnetic particles.
[0036] (2) Preparation of matrine magnetic slow-release granules: prepare 12 mL of 1% acetic acid aqueous solution, add 0.24 g of chitosan to make it naturally peptized, and repeatedly spray or wrap the above-mentioned chitosan acetic acid solution in Sophora flavescens for 13 times The surface of the alkali-loaded magnetic granules is sieved with a 50-mesh sieve, sized, and dried at a constant temperature of 50°C to obtain the matrine magnetic sustained-release granules.
[0037] (3) Preparation of matrine magnetic sus...
Embodiment 3
[0039] (1) Preparation of matrine drug-loaded magnetic particles: weigh 0.4 g of microcrystalline cellulose, Fe 3 o 4 After mixing 0.12g of magnetic nanoparticles and 0.1g of matrine, add 0.025g / mL chitosan in 2mL of 1% acetic acid solution, stir evenly, and make a soft material. Pass through a 65-mesh sieve to granulate, dry at a constant temperature of 50°C, sieve through a 65-mesh sieve, and granulate to obtain matrine drug-loaded magnetic particles.
[0040] (2) Preparation of matrine magnetic slow-release granules: prepare 6 mL of 1% acetic acid aqueous solution, add 0.15 g of chitosan to make it naturally peptized, and repeatedly spray or wrap the above-mentioned chitosan acetic acid solution in Sophora flavescens for 13 times The surface of the alkali-loaded magnetic granules is sieved with a 50-mesh sieve, sized, and dried at a constant temperature of 50°C to obtain the matrine magnetic sustained-release granules.
[0041] (3) Preparation of matrine magnetic sustaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com