Neostigmine bromide slow release preparation and its preparation method
A kind of bromine neostigmine and slow-release technology, which is applied in the field of bromine neostigmine sustained-release tablet and its preparation field, can solve the problems such as no research report of bromine neostigmine sustained-release tablet, and avoid instability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
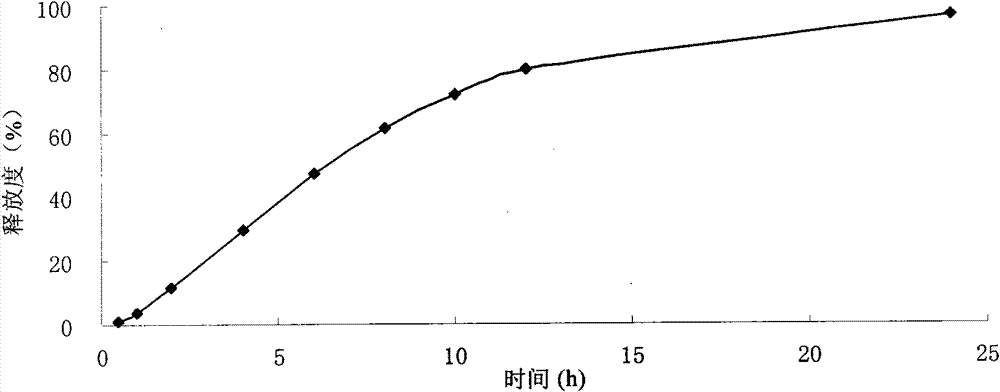
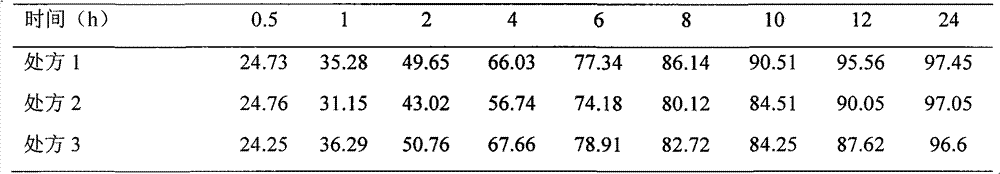
Image
Examples
Embodiment 1
[0056] The tablet core is a skeleton tablet, including the following components, and its weight composition is as follows:
[0057] Neostigmine bromide 750 parts
[0058] HPMC K4M 2000 copies
[0059] Lactose 1000 parts
[0060] Magnesium stearate 3333 parts
[0062] 125 parts of 95% ethanol solution
[0063] The prescription of coating solution I, coating solution I comprises following each component, and its weight composition is:
[0064] Cellulose acetate 250 parts
[0065] PEG6000 15 parts
[0066] Neostigmine Bromide 8 parts
[0067] Acetone 6667 parts
[0068] 1667 parts of absolute ethanol
[0069] The prescription of coating liquid II, coating liquid II comprises following each component, and its weight composition is:
[0070] 13 parts cellulose acetate
[0071] PEG6000 1 part
[0072] Acetone 417 parts
[0073] The preparation method comprises the following steps: (1) tablet core preparation: take by weighing neostigmine b...
Embodiment 2
[0076] The tablet core is a skeleton tablet, including the following components, and its weight composition is as follows:
[0077] Neostigmine bromide 700 parts
[0078] HPMC K15M 2000 copies
[0079] Lactose 1000 parts
[0080] Magnesium stearate 3000 parts
[0082] 125 parts of 95% ethanol solution
[0083] The prescription of coating solution I, coating solution I comprises following each component, and its weight composition is:
[0084] Ethyl cellulose 250 parts
[0085] PEG4000 15 parts
[0086] Neostigmine Bromide 8 parts
[0087] The prescription of coating liquid II, coating liquid II comprises following each component, and its weight composition is:
[0088] 13 parts ethyl cellulose
[0089] PEG6000 1 part
[0090] Preparation method is basically the same as embodiment 1. The difference is that the coating is coated with an aqueous dispersion of the coating liquid.
Embodiment 3
[0092] The tablet core is a skeleton tablet, including the following components, and its weight composition is as follows:
[0093] Neostigmine bromide 650 parts
[0094] HPMC K100M 2000 copies
[0095] Lactose 1000 parts
[0096] Magnesium stearate 3000 parts
[0098] 95% ethanol solution 110 parts
[0099] The prescription of coating solution I, coating solution I comprises following each component, and its weight composition is:
[0100] Cellulose acetate 210 parts
[0101] PEG6000 15 parts
[0102] Neostigmine bromide 5 parts
[0103] Acetone 6000 parts
[0104] 1500 parts of absolute ethanol
[0105] The prescription of coating liquid II, coating liquid II comprises following each component, and its weight composition is:
[0106] 13 parts cellulose acetate
[0107] PEG6000 1 part
[0108] Acetone 400 parts
[0109] Preparation method is basically the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com