Lappaconitine hydrobromide orally disintegrating tablets and preparation method thereof
A kind of technology of urine hydrobromide and orally disintegrating tablets, which is applied in the direction of medical formula, medical preparations of non-active ingredients, pill delivery, etc., which can solve the problem of unsatisfactory taste masking effect, easy to affect drug dissolution, process and so on. Complicated and other issues, to achieve the effect of high product yield, fast drug dissolution, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
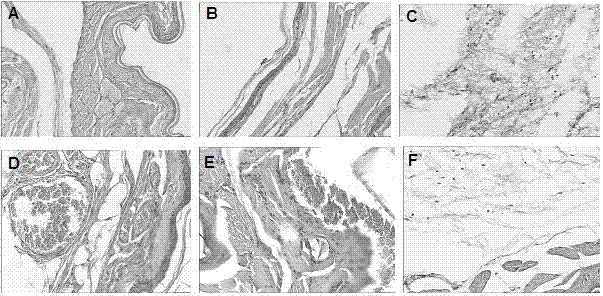
[0031] Embodiment 1. Preparation (wet granulation and tablet compression method) and inspection of orally disintegrating tablets of homogenate hydrobromide without gastric-soluble acrylic resin component in the prescription
[0032]Prescription: 5g of homogenate hydrobromide, 4.8g of cross-linked polyvinylpyrrolidone, 15g of microcrystalline cellulose, 30g of mannitol, 5g of aspartame, 30g of water, 0.5g of magnesium stearate, made into 1000 tablets in total .
[0033] Preparation process: mix homogenine hydrobromide, cross-linked polyvinylpyrrolidone, microcrystalline cellulose, mannitol and aspartame, pass through a 100-mesh sieve twice, add water to make a soft material, and granulate with a 20-mesh sieve. Dry at 60°C until the water content is less than or equal to 3%, then add magnesium stearate, mix well, and press into tablets to control the hardness of the tablet to 3Kg to obtain the orally disintegrating tablet of urine hydrobromide.
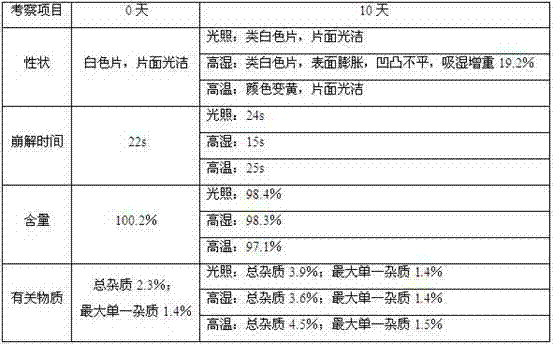
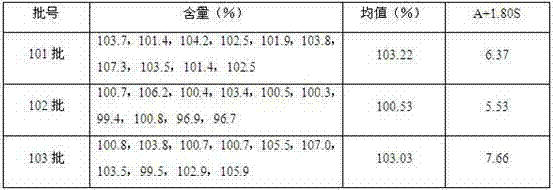
[0034] According to the above ...
Embodiment 2
[0063] Embodiment 2. Preparation of orally disintegrating tablets of homogenate hydrobromide without gastric-soluble acrylic resin component in the prescription (dry granulation and tablet compression method)
[0064] Prescription: 10g of homogenate hydrobromide, 5g of cross-linked polyvinylpyrrolidone, 40g of modified starch-1500, 30g of sorbitol, 2g of glycyrrhizin, 5g of thaumatin, 1g of mint essence, 1g of magnesium stearate, talc Powder 1g, made into 1000 tablets in total.
[0065] Preparation process: mix homogenate hydrobromide, cross-linked polyvinylpyrrolidone, modified starch-1500, sorbitol, glycyrrhizin, thaumatin and mint essence, pass through a 120-mesh sieve for 3 times, and dry granulate , pulverized, crossed 20 mesh sieves, then added magnesium stearate and talcum powder, mixed evenly, compressed into tablets, and the tablet hardness was controlled to be 5Kg, to obtain the orally disintegrating tablets of uricine hydrobromide.
[0066] Similar to the orally d...
Embodiment 3
[0067] Embodiment 3. Preparation of orally disintegrating tablets of homogenate hydrobromide without gastric-soluble acrylic resin component in the prescription (direct compression method)
[0068] Prescription: 5g of urine hydrobromide, 3g of croscarmellose sodium, 15g of microcrystalline cellulose, 15g of sorbitol, 2g of glycyrrhizin, 2g of aspartame, 1g of citric acid, 1g of orange essence, Magnesium stearate 0.5g, made into 1000 tablets in total.
[0069] Preparation process: mix homogenate hydrobromide, croscarmellose sodium, microcrystalline cellulose, sorbitol, glycyrrhizin, aspartame and chocolate essence, pass through 80-mesh sieve for 3 times, Then add magnesium stearate, mix evenly, and press into tablets to control the hardness of the tablet to 4Kg to obtain the orally disintegrating tablet of uricine hydrobromide.
[0070] Similar to the orally disintegrating tablet obtained in Example 1, the orally disintegrating tablet of homogenate hydrobromide prepared in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com