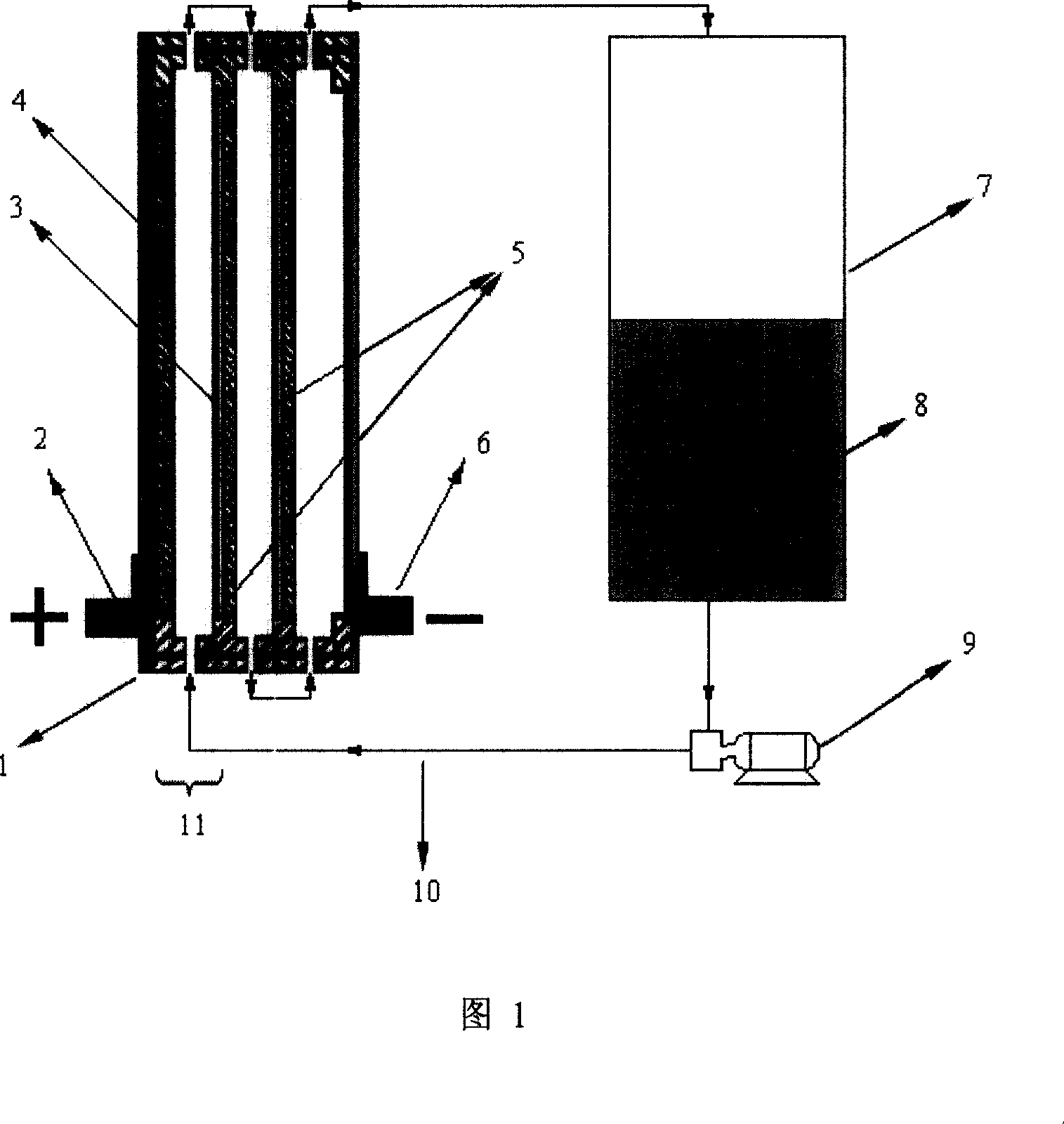
A Zn-Ni liquid battery
A flow battery, flow battery technology, applied in battery electrodes, alkaline batteries, battery pack components, etc., can solve the problems of reduced discharge rate, low performance stability, nickel electrode swelling, etc., to achieve high energy density and High power density, high energy utilization efficiency, and the effect of eliminating concentration polarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Positive electrode preparation
[0034]Mix the conductive agent carbonyl nickel powder and the binder CMC (sodium carboxymethylcellulose) at a mass ratio of 1:1, make a slurry with polyol and water, and apply it evenly on the perforated nickel-plated steel by wet squeegee method The belt is continuously dried in a vertical furnace to obtain a dried nickel substrate. The dried nickel substrate is sintered at 1000° C. for 10 minutes under a reducing atmosphere in a sintering furnace to obtain a sintered nickel substrate. The sintered nickel substrate is used as the cathode and electrolyzed in a slightly acidic aqueous solution containing nickel, cadmium, and cobalt salts. During the electrolysis process, the nickel, cadmium, and cobalt are controlled to form hydroxide precipitates that are co-deposited in the micropores of the substrate, and no metal is precipitated. After the filling amount of the active material of the electrolytic substrate reaches the requirement, it...
example 2
[0036] Negative electrode preparation
[0037] Roll the steel strip to about 50 μm, and use a punching machine to make suitable holes on the steel strip to obtain a perforated steel strip. Generally, the porosity is required to be 10-50%. After proper cutting, the perforated steel strip can be used as negative electrode current collector in zinc-nickel flow battery, and it can also be used after nickel plating.
example 3
[0039] Zinc-nickel flow battery assembly
[0040] Arrange the positive and negative electrodes in a suitable container in a positive and negative manner, reserve a certain gap between the electrodes, set up an electrolyte circulation channel in the gap and seal it into a battery cell, connect the electrolyte pipeline, pump and electrolyte storage Can. While the electrolyte is flowing, charging (the negative electrode deposits zinc) and discharging (the negative electrode zinc redissolves into the electrolyte) can take place. The zinc-nickel redox flow battery prepared in this way can reach the level of all-vanadium redox flow battery in terms of specific energy and specific power, and has a higher cycle life.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com