Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
66results about How to "Recovery height" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
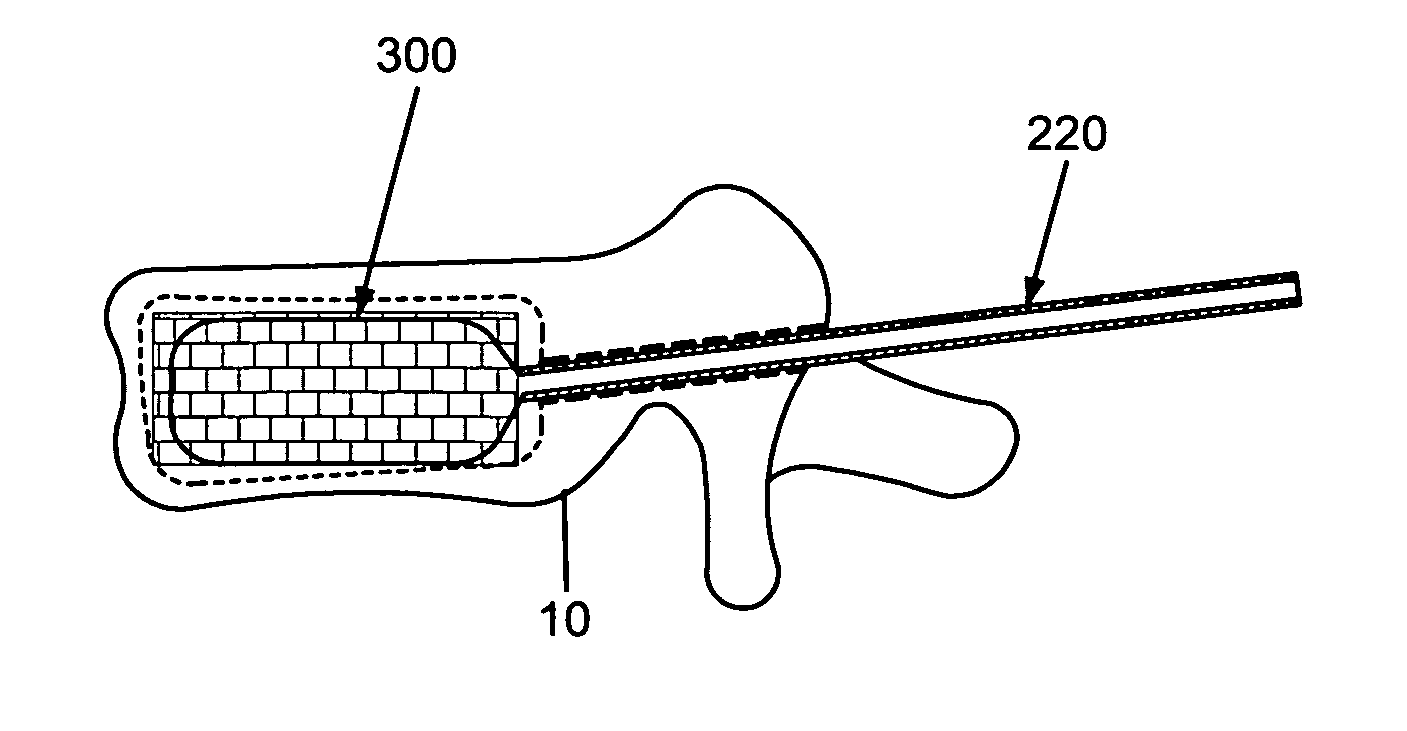
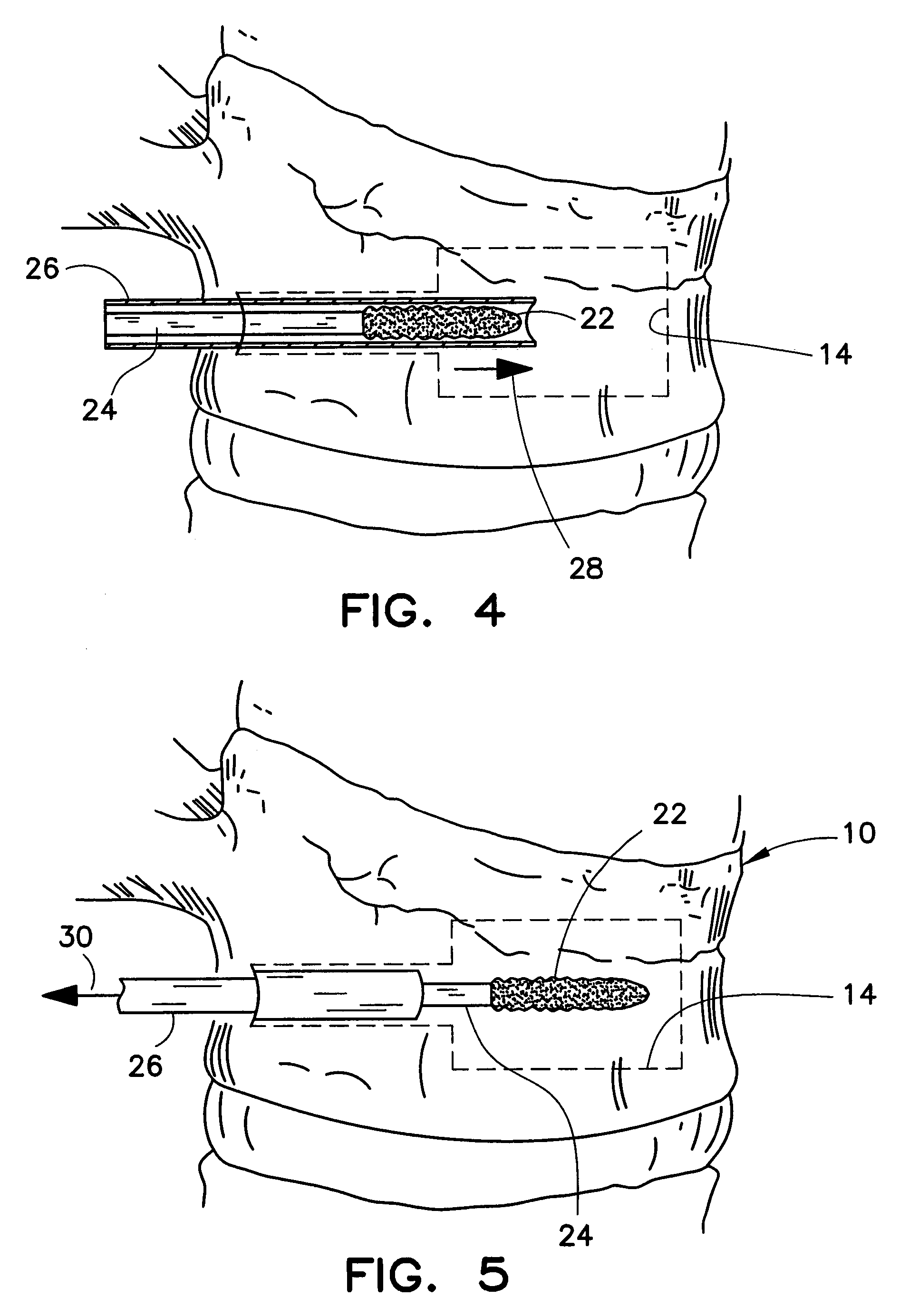
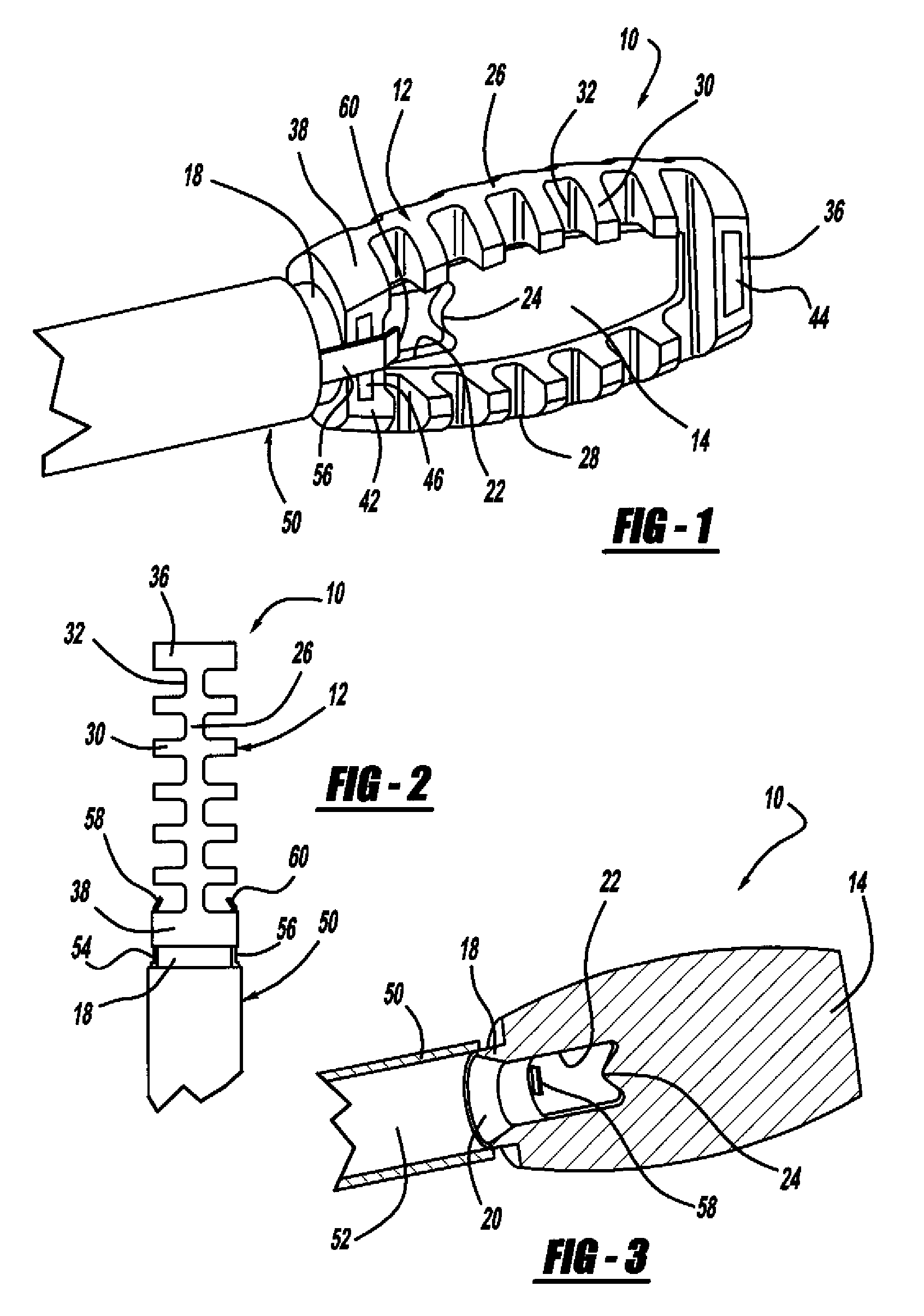
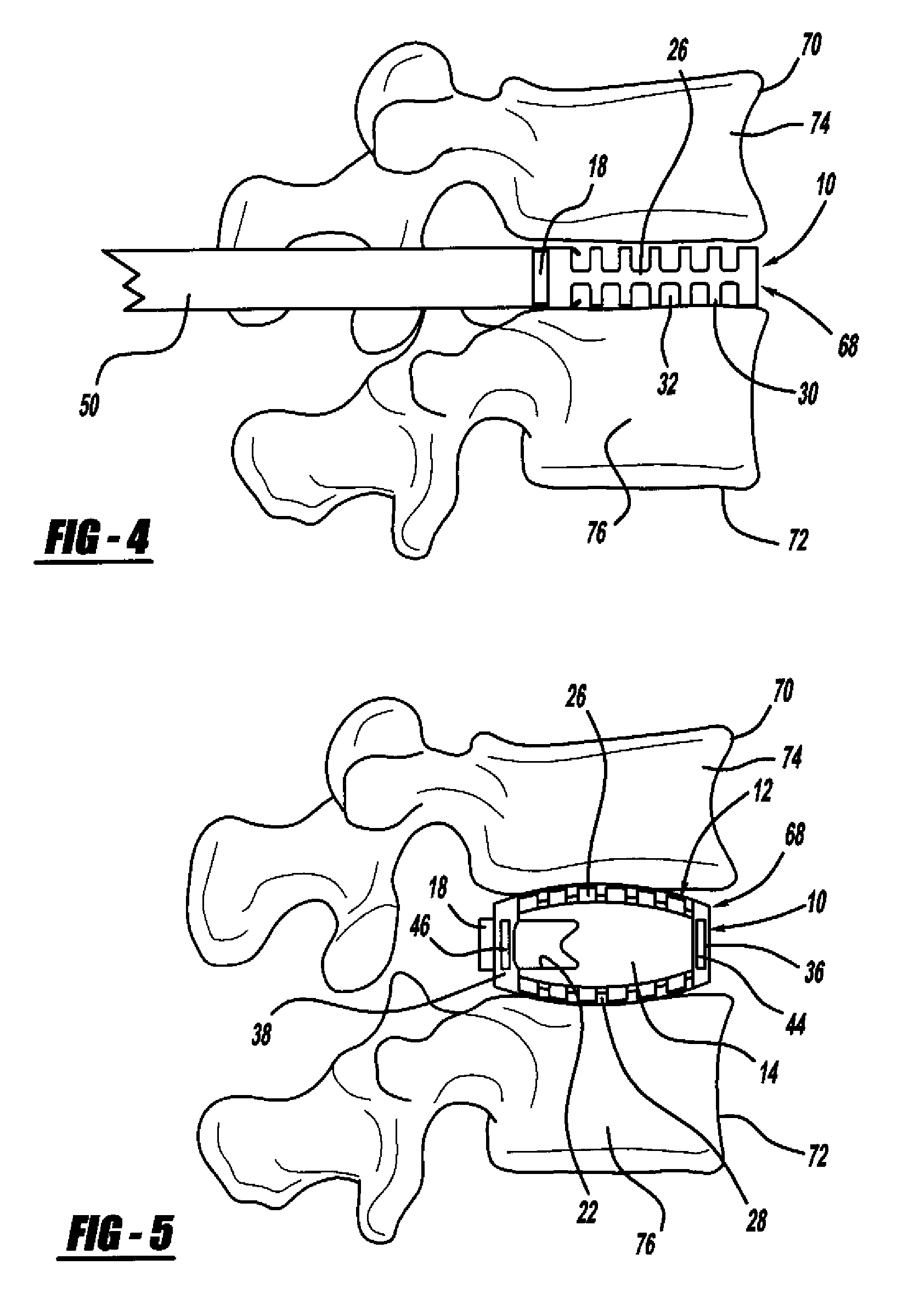
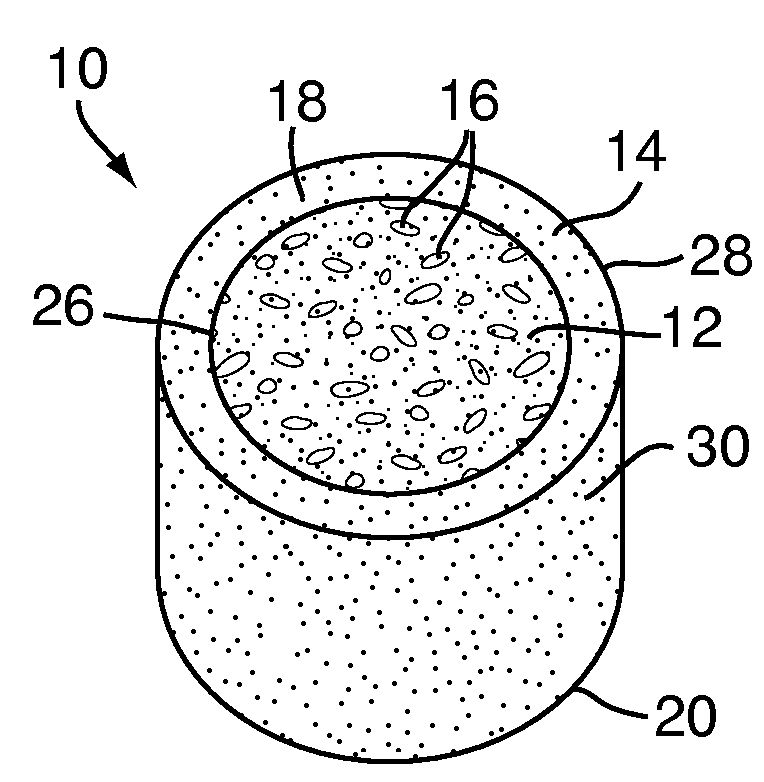
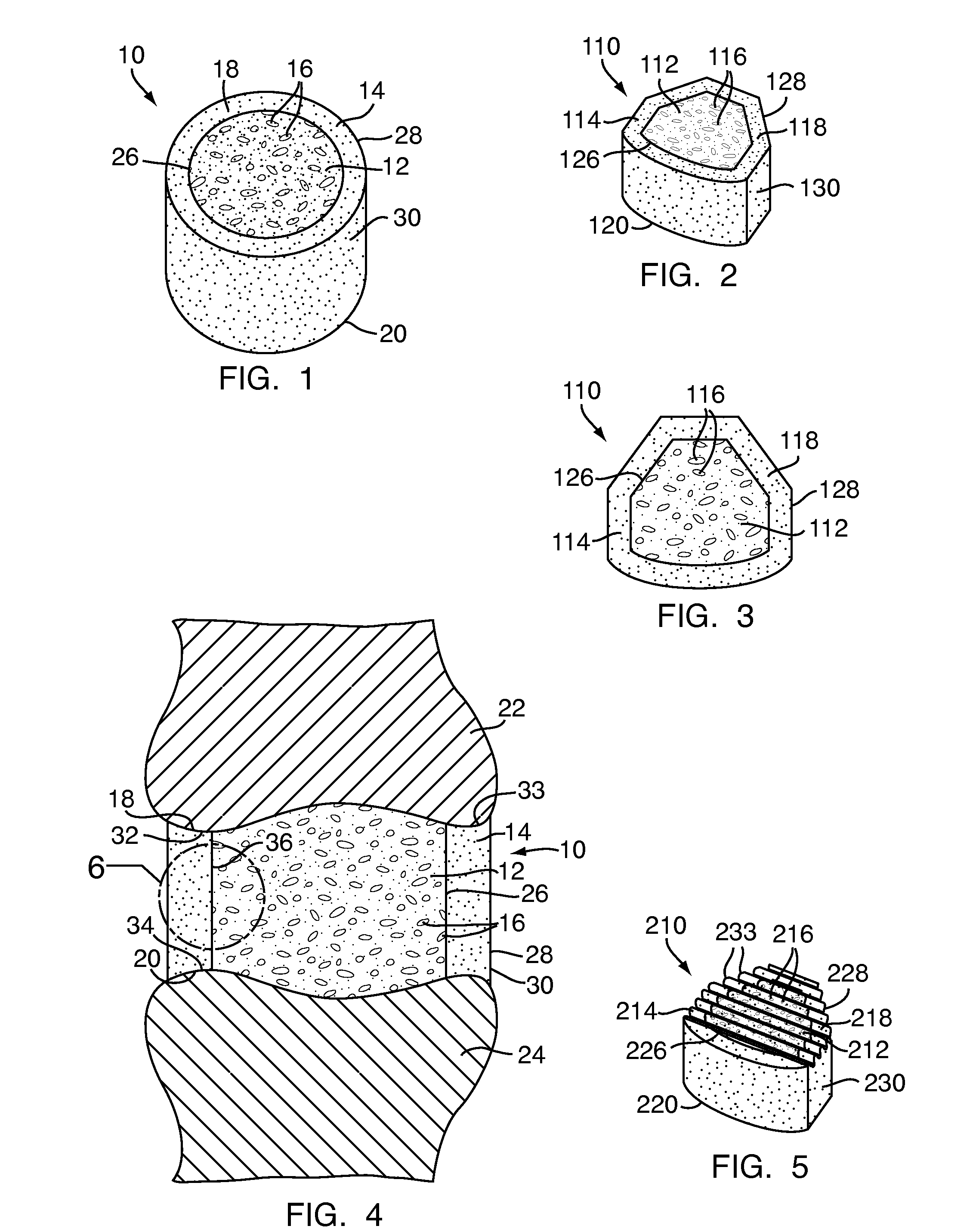
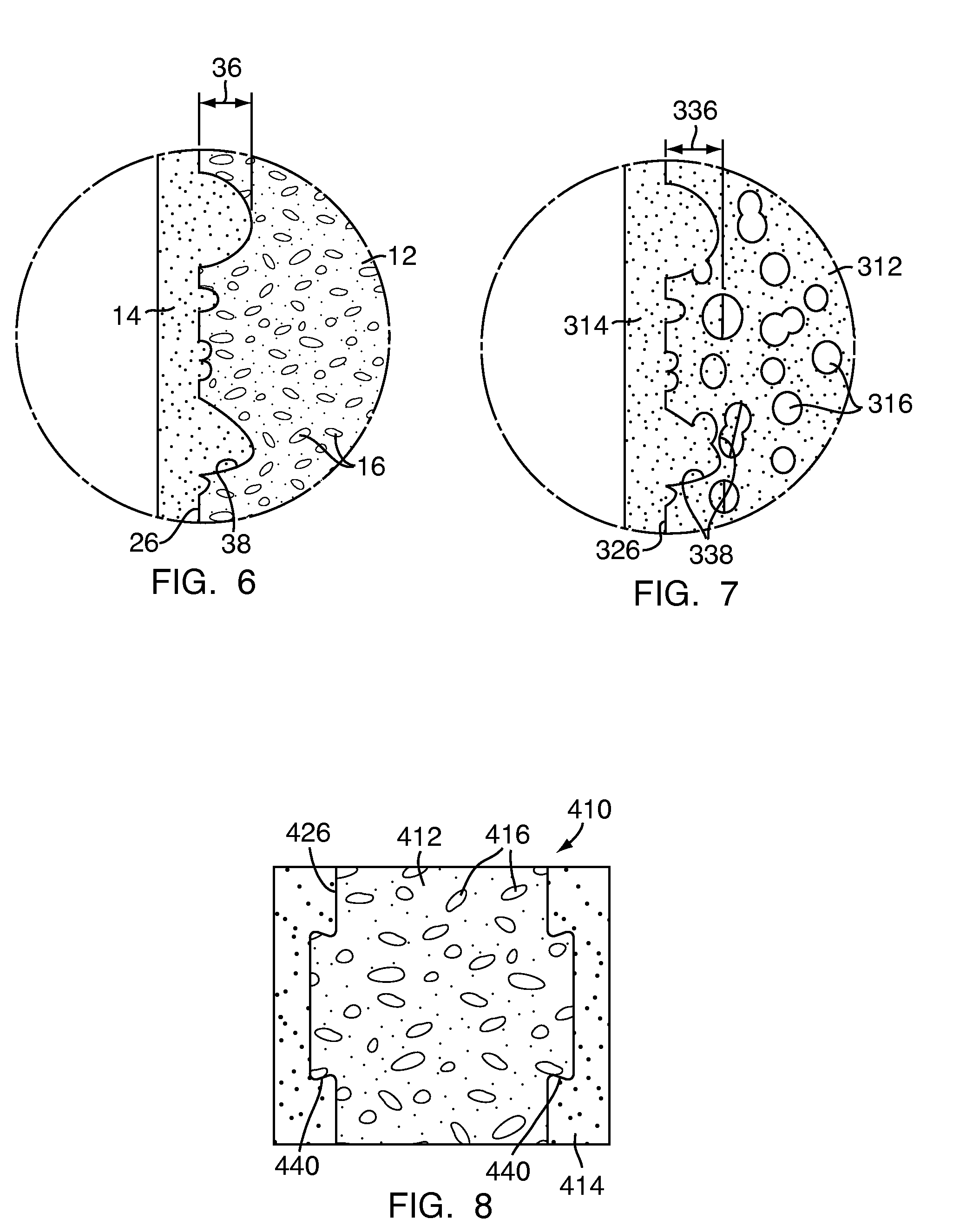
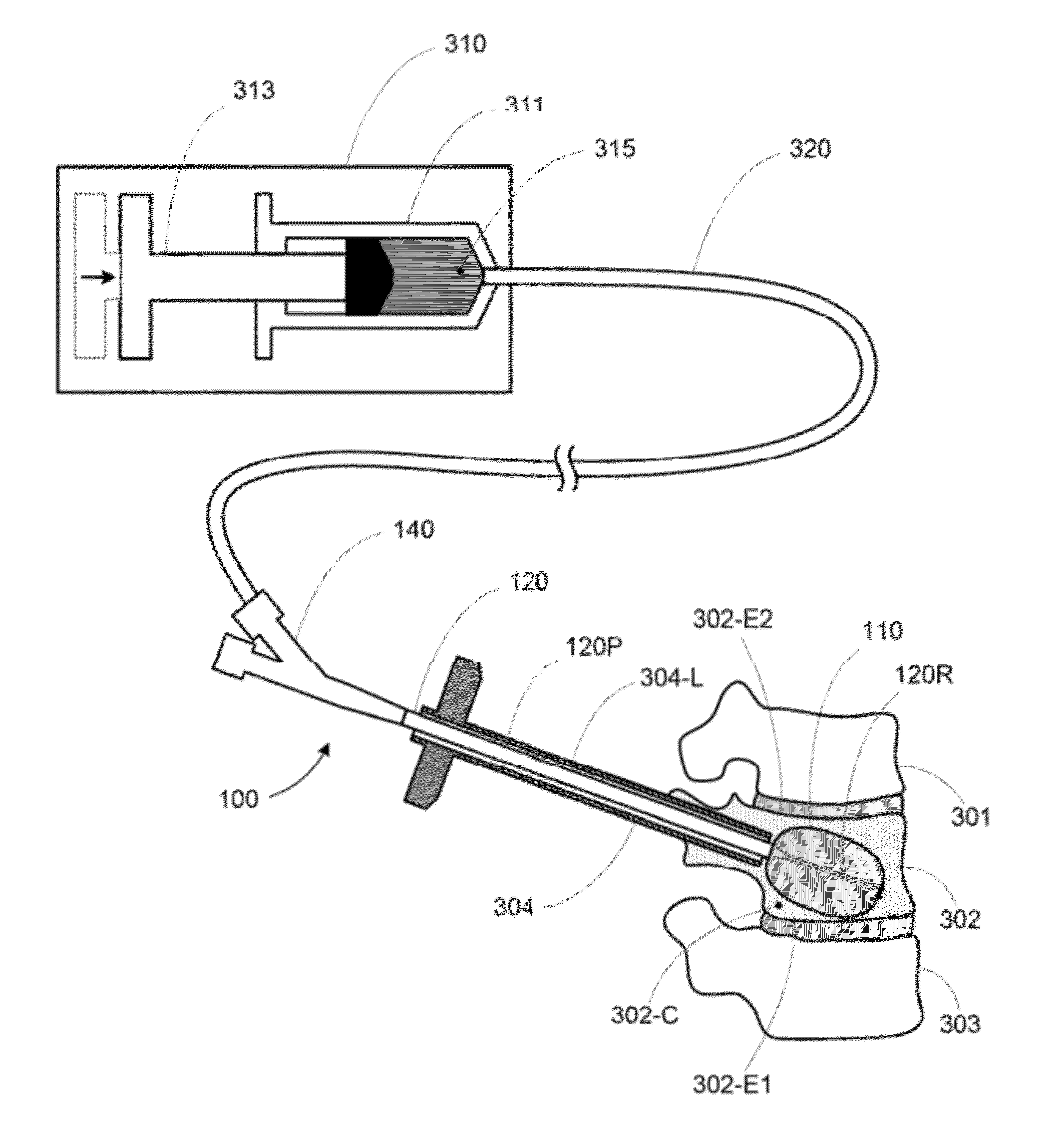
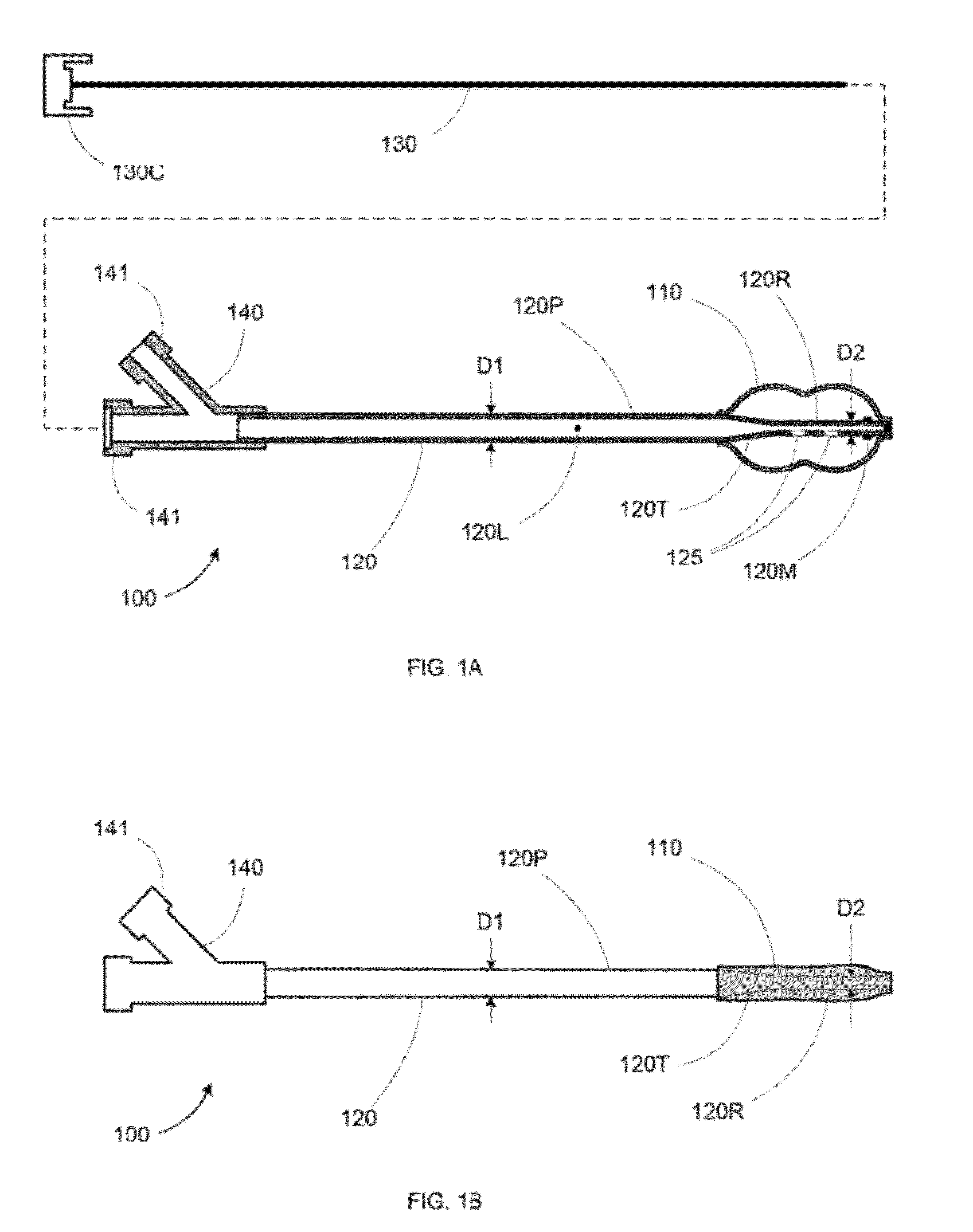
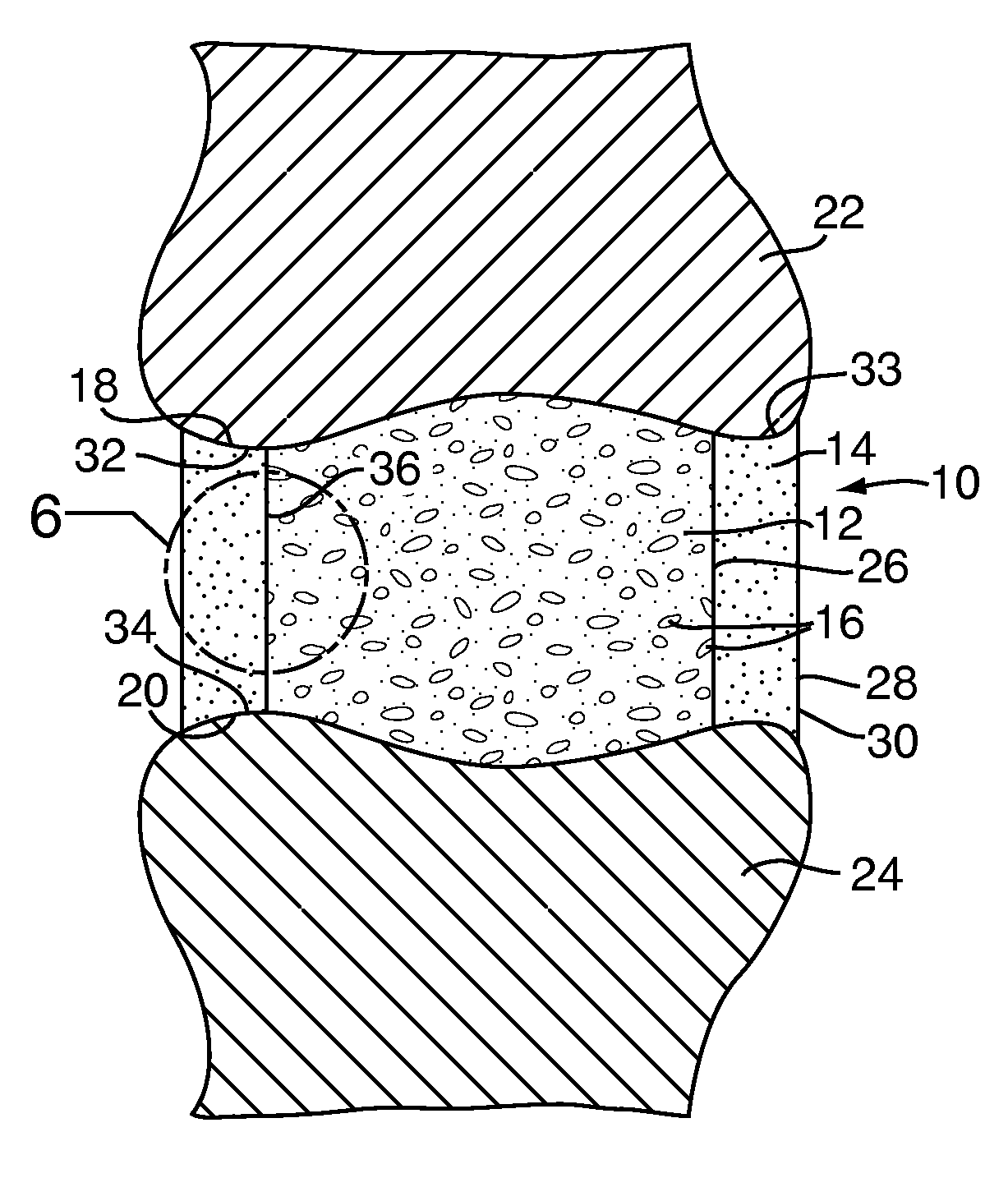
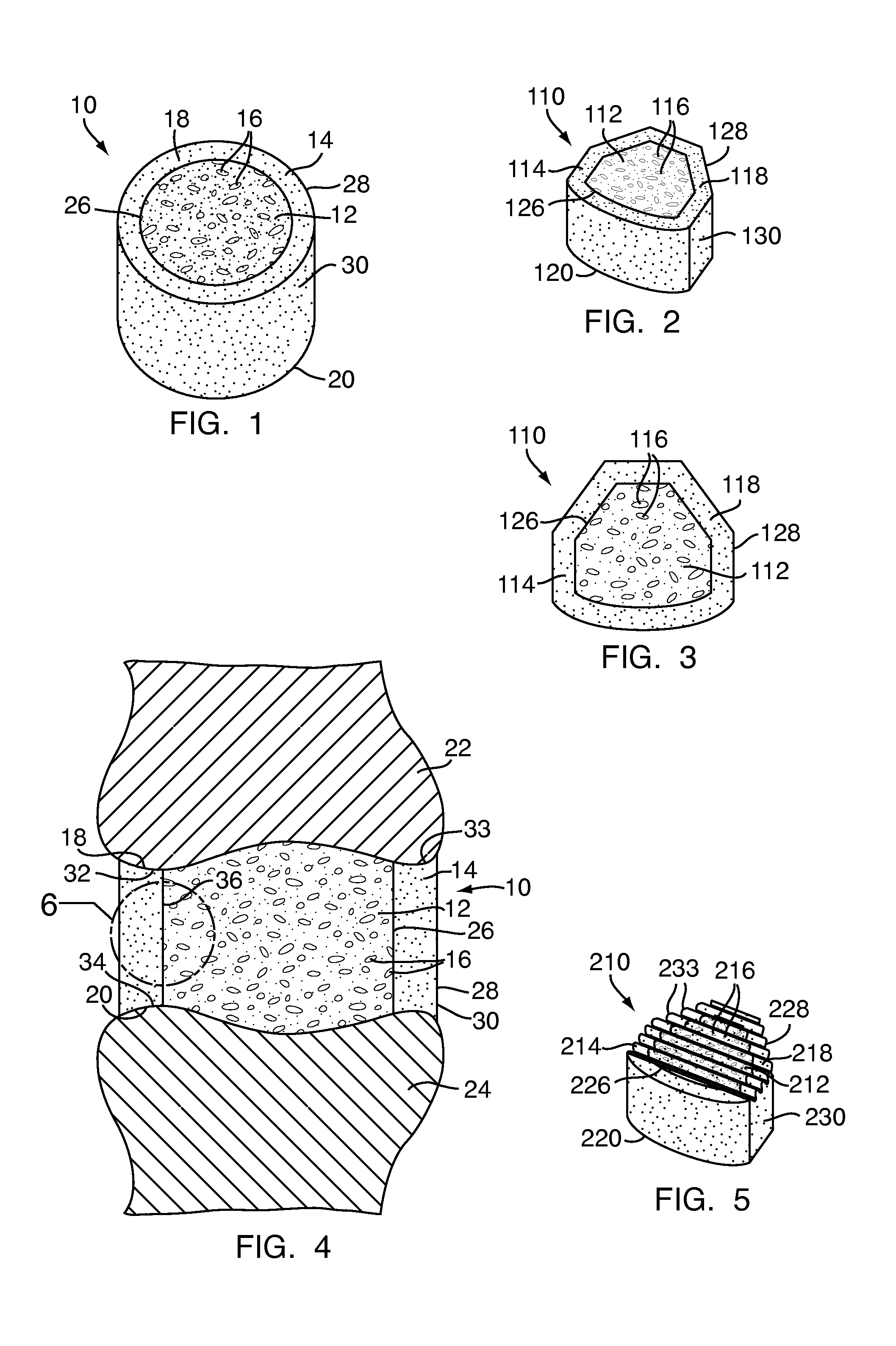
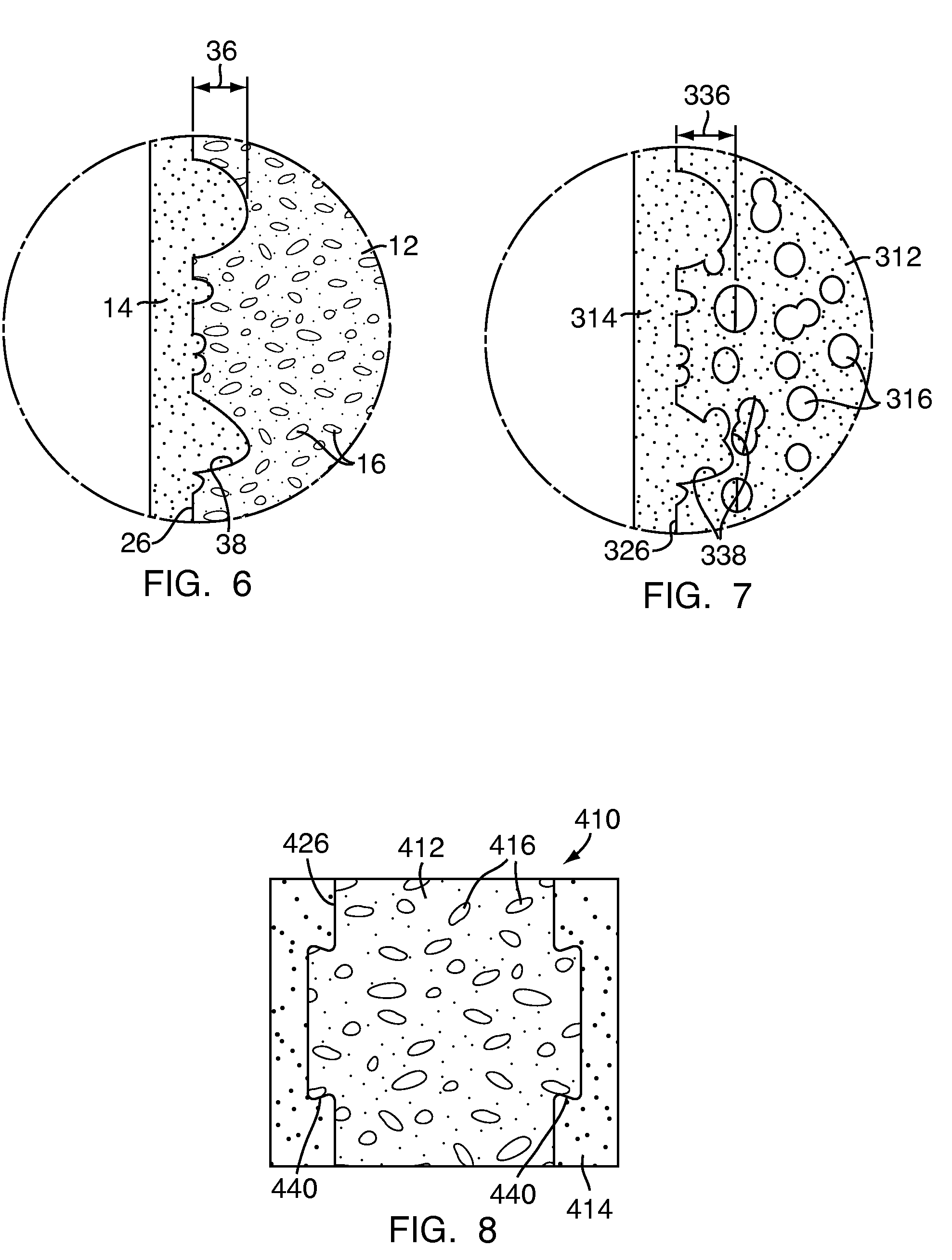
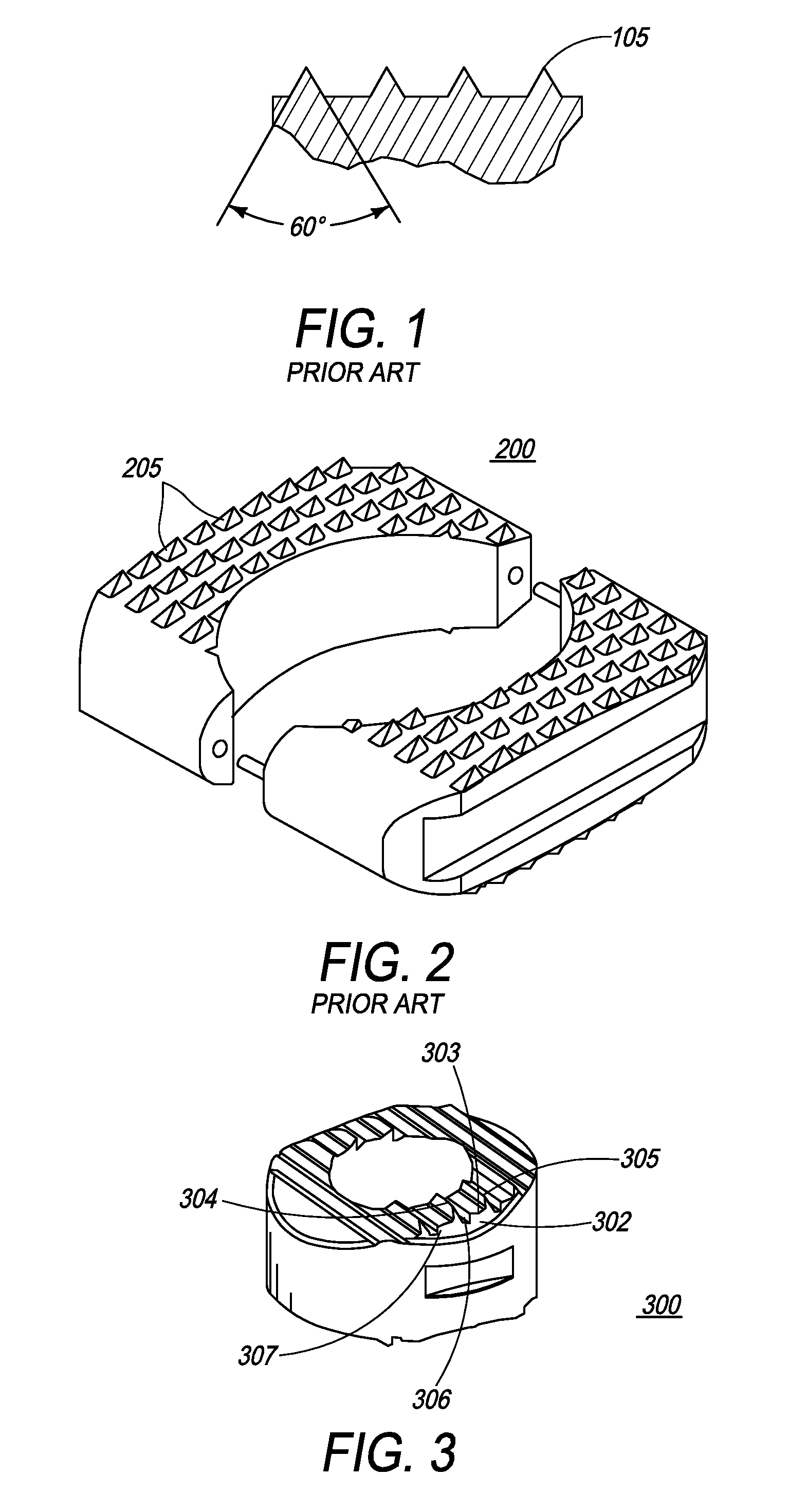
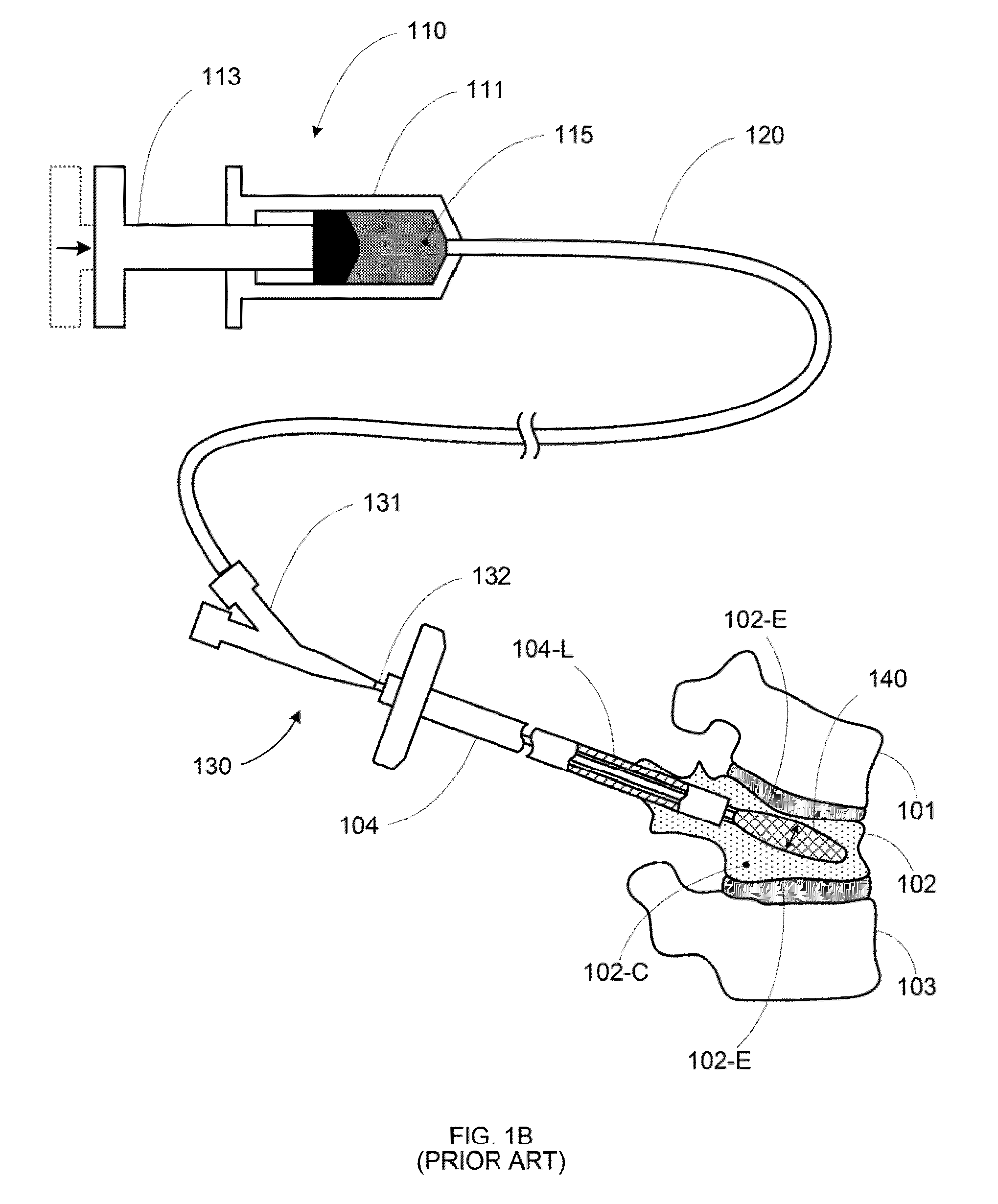
Apparatus and methods for treating bone
InactiveUS20070093899A1Minimally augmentationRecovery heightInternal osteosythesisCannulasInsertion stentBone implant
Implants and methods for minimally invasive augmentation and repositioning of vertebrae may comprise one or more expandable members, e.g., stents, implants, surrounding a balloon-tipped catheter or other expansion device, inserted into a vertebral body or other bone. Expansion of the expandable member within the vertebral body or other bone may reposition the fractured bone to a desired height and augment the bone to maintain the desired height. A bone cement or other filler can be added to further augment and stabilize the vertebral body or other bone.
Owner:SYNTHES USA
Vertebral device for restoration of vertebral body height
An intra-vertebral body height restoring device includes a body for insertion into an intra-vertebral space. The body includes top and bottom surfaces for engaging opposing vertebral surfaces defining the intra-vertebral space. The body includes at least two layers extending along a width of the body and having a fully expanded and fully collapsed height relative thereto. A reversible expansion mechanism selectively and reversely expands and collapse the height of the layers and including the fully expanded and collapsed heights to restore a selected height to the intra-vertebral space.
Owner:RICHELSOPH MARC E
Method and apparatus for treating a vertebral body
InactiveUS7931689B2Restore heightRecovery heightInternal osteosythesisBone implantBiomedical engineeringBone cement
An implantable container is used to stabilize or restore height in a vertebral body. After insertion the container is filled with a bone filler material such as bone cement.
Owner:SPINEOLOGY
Tissue distraction device
InactiveUS20050187558A1Recovery heightImprove the forceInternal osteosythesisBone implantDistractionTissue surface
Owner:SPINEWAVE
Tissue distraction device
ActiveUS20050171552A1Process stabilityEasy to moveInternal osteosythesisBone implantDistractionTissue surface
Owner:SPINEWAVE
Minimally Invasive Interbody Device Assembly
InactiveUS20080172128A1Restore heightEasy to placeInternal osteosythesisJoint implantsIntervertebral diskInstrumentation
A minimally invasive interbody device assembly that includes an interbody device that restores the disc space height between two vertebrae and an instrument detachably coupled to the interbody device for positioning the device in the disc space and delivering bone material to the disc space that is distributed on both sides of the interbody device. The device is inserted into the disc space using the instrument in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated by the instrument so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then forced down the instrument so that the bone graft material is distributed on both sides of the device. The instrument is then detached from the device.
Owner:THOMPSON MIS
Spinal dynamic stabilization device
InactiveUS20090112266A1Reduce complexityShorten the timeSuture equipmentsInternal osteosythesisTranspedicular fixationBiomedical engineering
A spinal dynamic stabilization device for maintaining an anatomical height between two adjacent vertebras is provided. Each vertebra includes a spinous process and two symmetric pedicles. The spinal dynamic stabilization device includes a supporting member, at least one anchoring member, and at least one connecting member. The supporting member is disposed between the spinous processes. The anchoring member is fixed in one of the vertebra via one of the pedicles. The connecting member connects the supporting member to the anchoring member, fixing a relative position between the supporting member and the anchoring member, further fixing a relative position between the vertebras.
Owner:IND TECH RES INST
Minimally Invasive Interbody Device
ActiveUS20080172127A1Restore heightDisc space heightInternal osteosythesisEar treatmentBone graft materialsVertebra
An interbody device that restores the disc space height between two vertebrae during spinal fusion surgery. The device includes a center plate surrounded by a perimeter portion that combine to have a relatively flat configuration in one dimension and relatively wide configuration in a perpendicular dimension. After the disc space has been cleared, the device is inserted into the disc space in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then introduced through a fill tube coupled to the device so that the bone graft material is distributed on both sides of the center plate and into the disc space.
Owner:THOMPSON MIS
Method for fabricating a multi-density polymeric interbody spacer
InactiveUS20110012280A1Restore heightPromote bone fusionEye surgerySurgeryPorosityUltimate tensile strength
A multi-density polymeric interbody spacer formed from biocompatible material for osteoconductivity includes multiple density regions of different porosity to provide both strength and osteoconductivity. An interface region is formed between the density regions to provide both direct adhesion and mechanical interlocking between the different density regions to increase the strength of the multi-density polymeric interbody spacer. A method for forming the multi-density polymeric interbody spacer includes curing a first density region to achieve a first target porosity. A second density region may then be molded to the first density region to achieve a second target porosity. A portion of the second density region partially flows into pores of the first density region, providing direct adhesion and mechanical interlocking between the first and second density regions.
Owner:DOCTORS RES GROUP
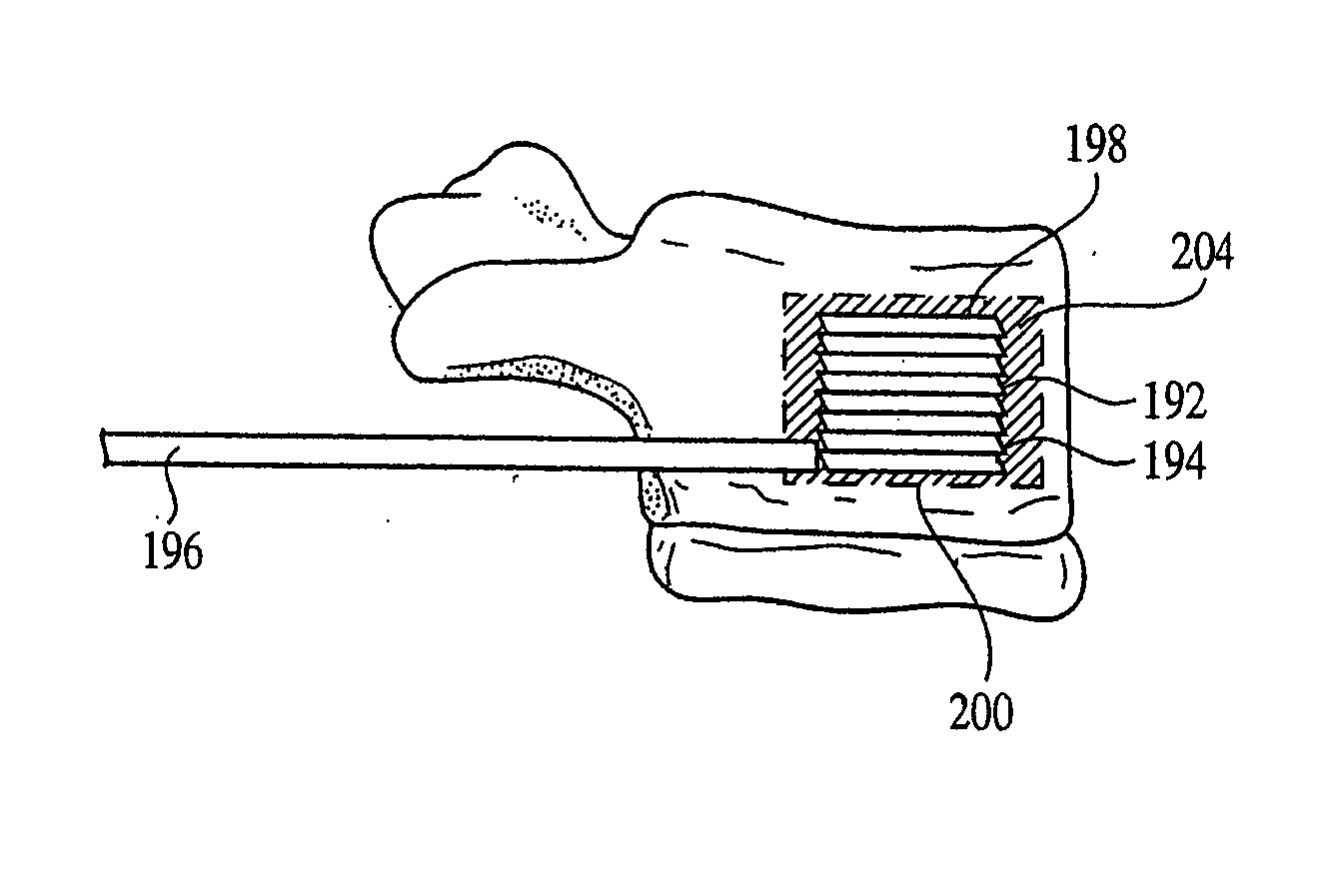
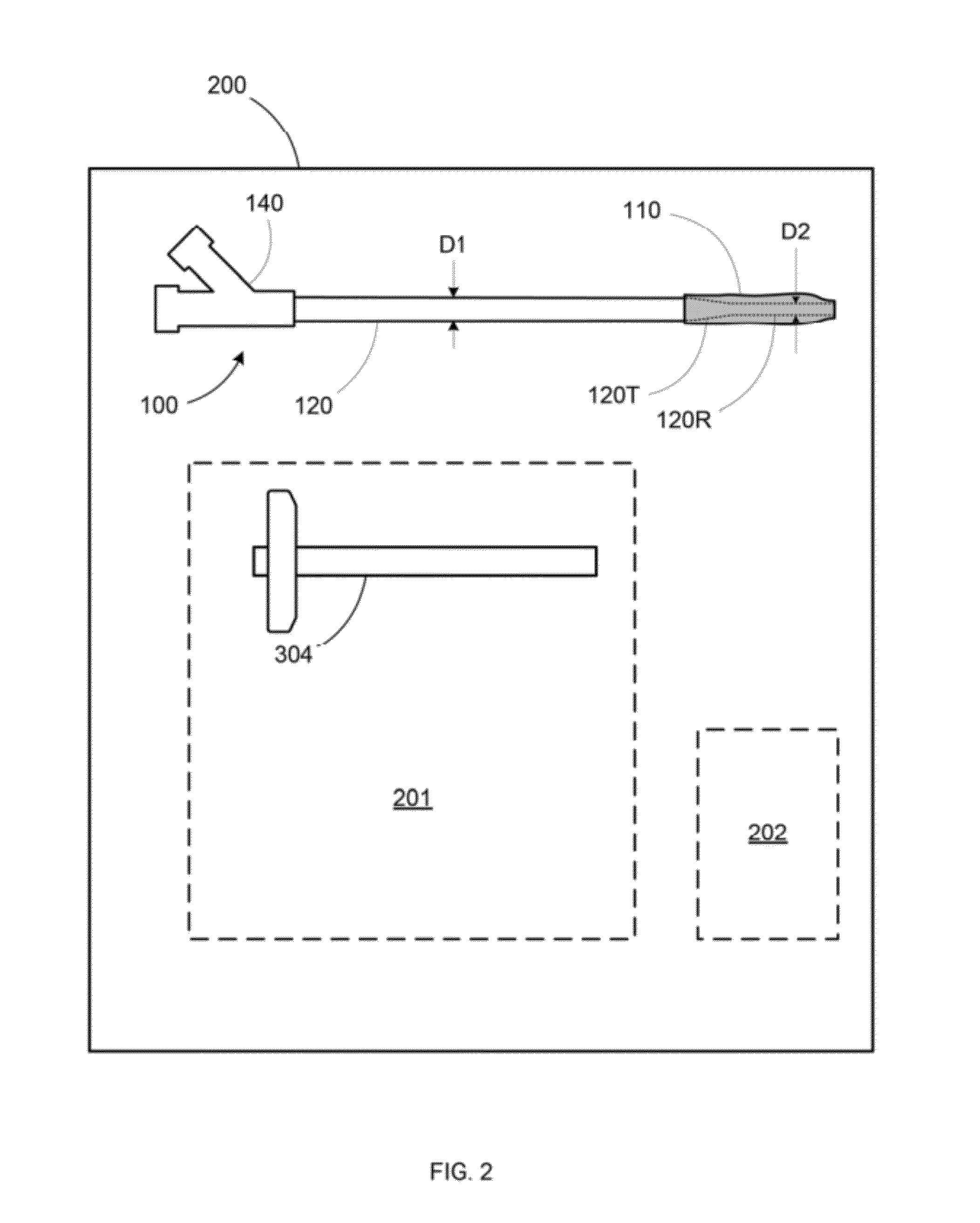
Low cost low profile inflatable bone tamp
ActiveUS20120259375A1Great distal expansionRaise the possibilityDiagnosticsBlunt dissectorsBalloon catheterSurgical procedures
An inflatable bone tamp for performing a minimally invasive surgical procedure includes a shaft having a primary region and a reduced diameter region, and an inflatable structure surrounding at least a portion of the reduced diameter region. The reduced diameter region of the shaft allows the deflated size of the inflatable structure to be minimized, while at the same time eliminating the need for the conventional dual lumen balloon catheter construction.
Owner:KYPHON
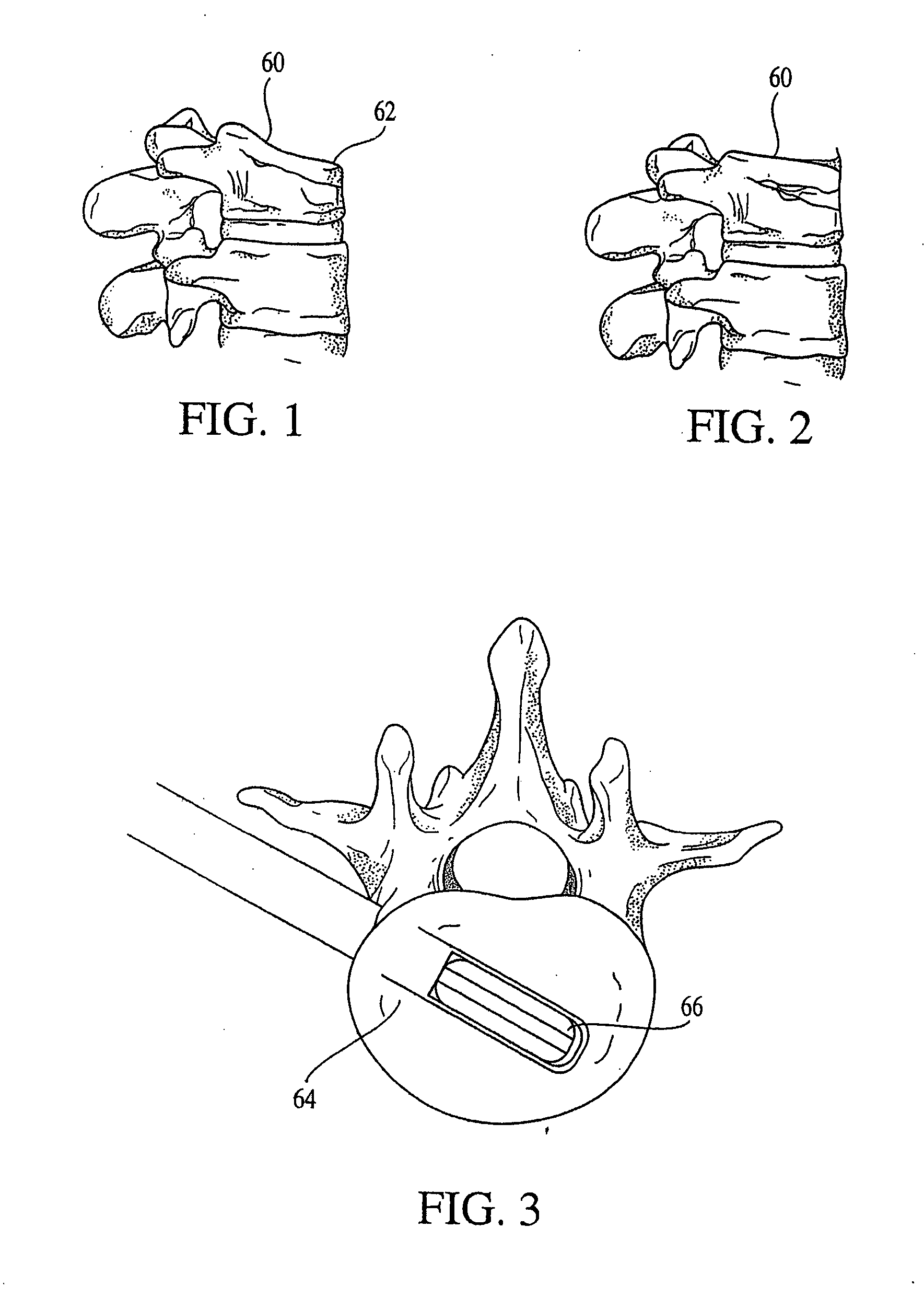
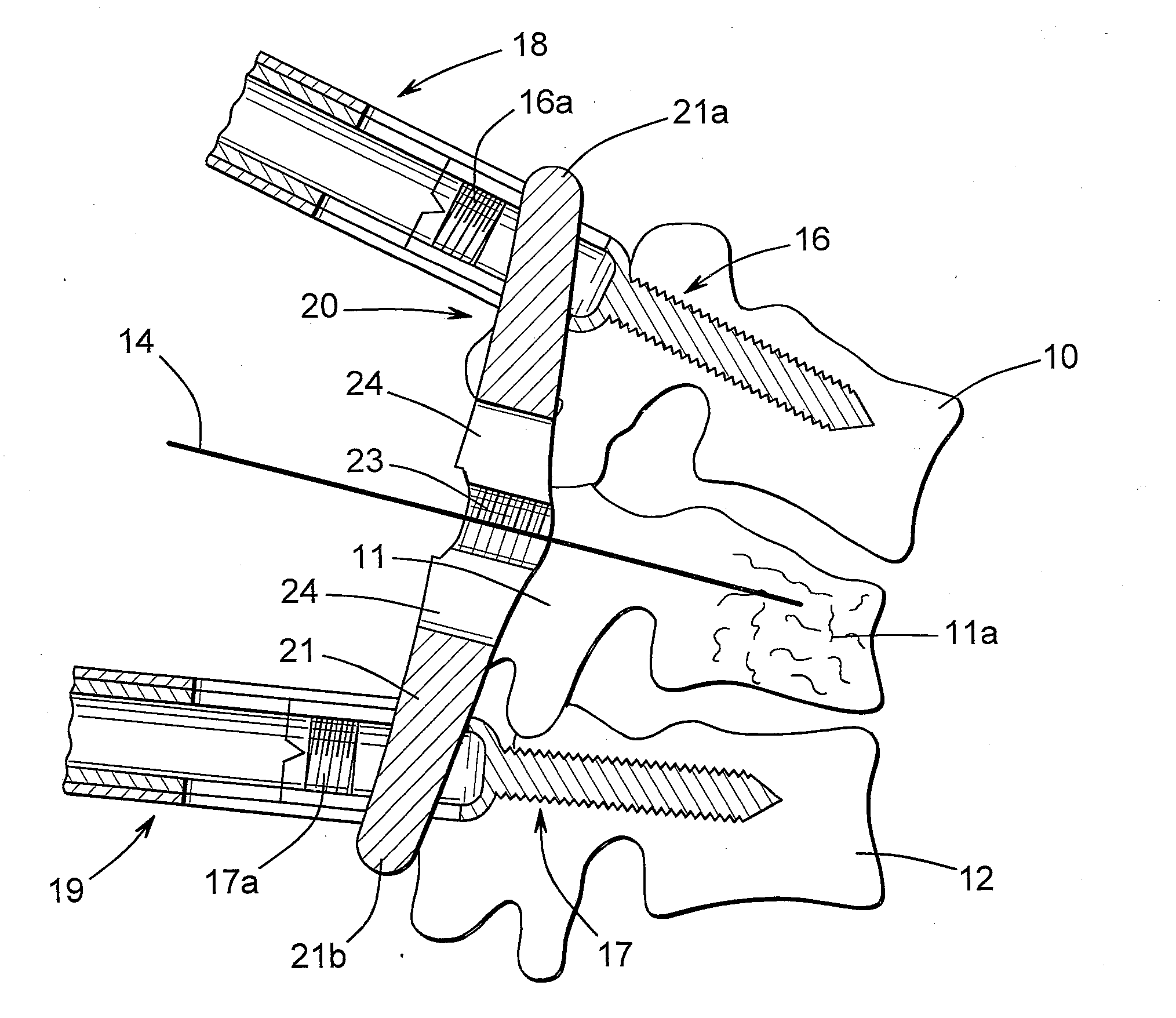
Posterior fixation device for percutaneous stabilization of thoracic and lumbar burst fractures
InactiveUS20100082066A1High strengthImprove stabilityInternal osteosythesisJoint implantsVertebraFastener
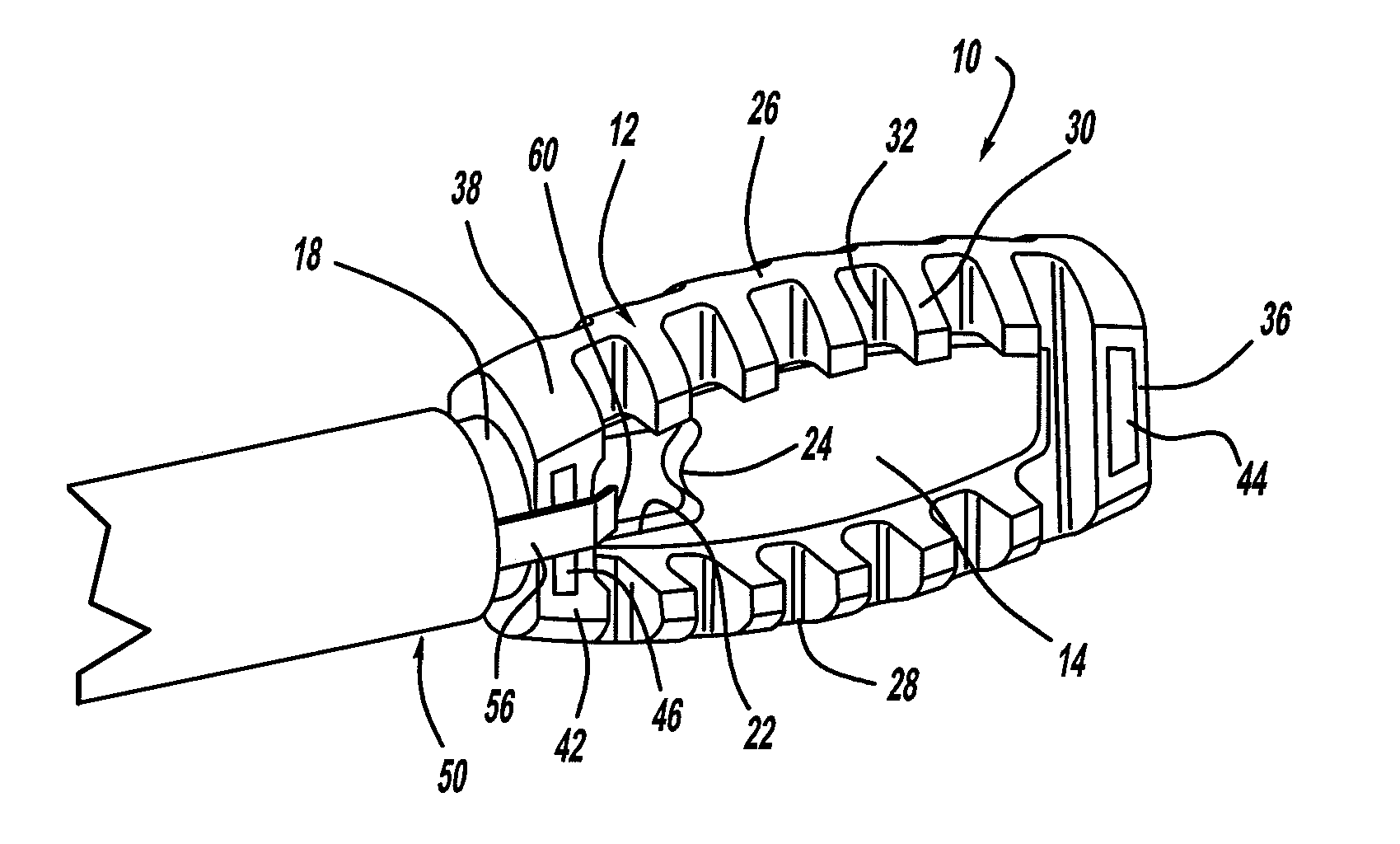
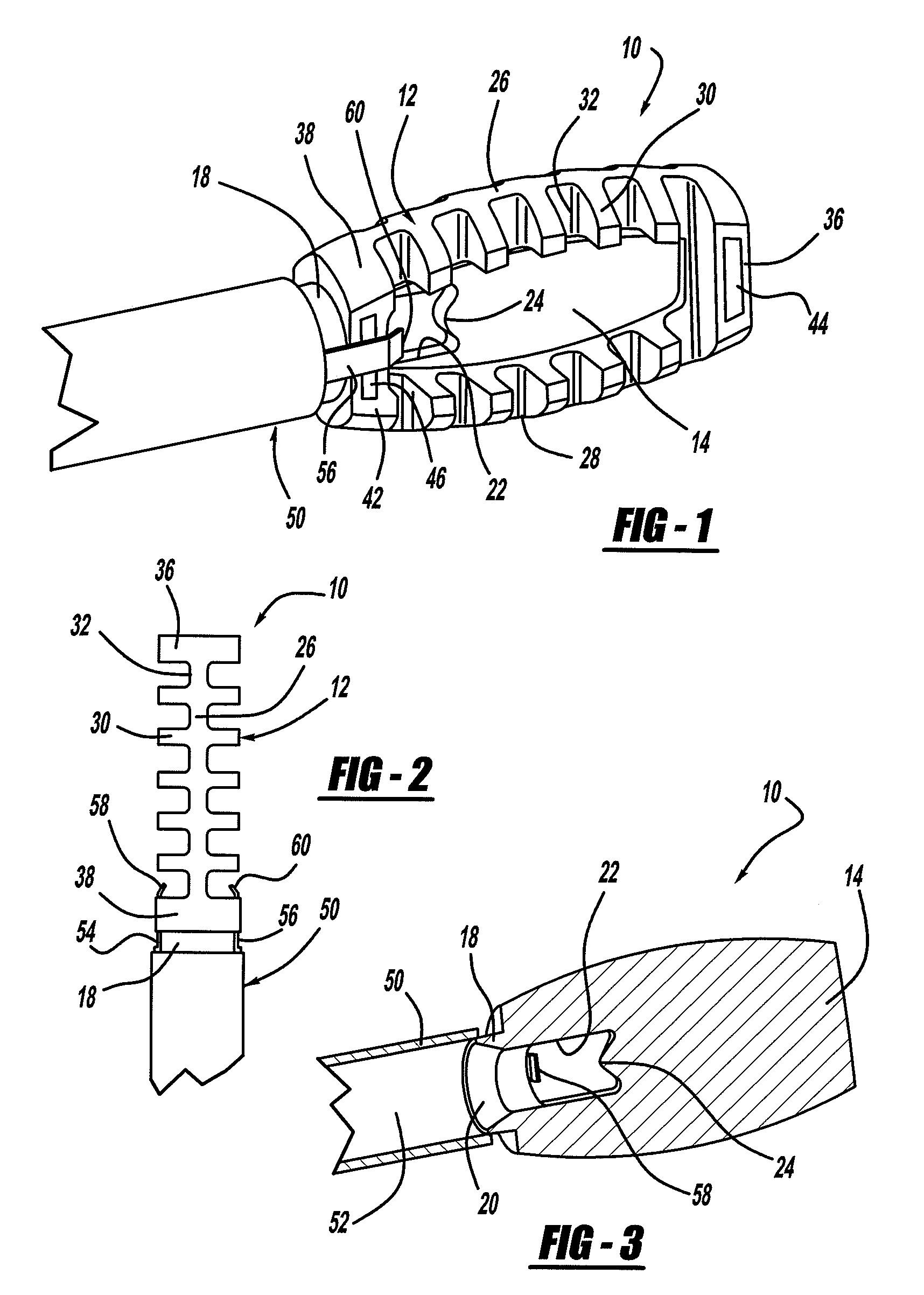
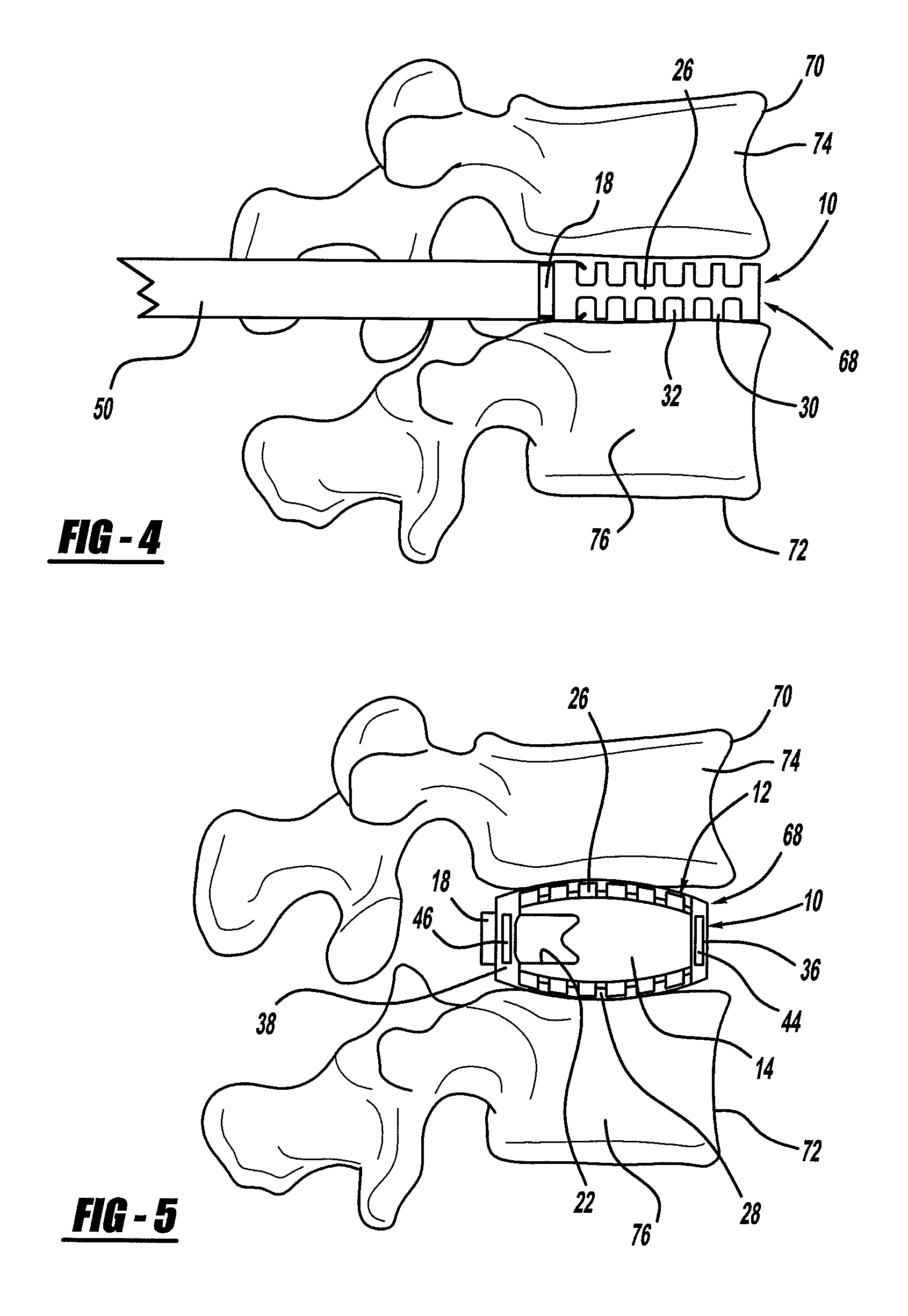
A spinal fixation device includes a fixation rod including a body having an aperture formed therethrough. First and second vertebral fasteners are secured to the body and are adapted to be secured to respective vertebrae. A third vertebral fastener extends through the aperture in the body of the fixation rod and adapted to be secured to a vertebra.
Owner:UNIVERSITY OF TOLEDO
Minimally invasive interbody device
ActiveUS7824427B2Disc space heightRecovery heightInternal osteosythesisJoint implantsIntervertebral diskVertebra
An interbody device that restores the disc space height between two vertebrae during spinal fusion surgery. The device includes a center plate surrounded by a perimeter portion that combine to have a relatively flat configuration in one dimension and relatively wide configuration in a perpendicular dimension. After the disc space has been cleared, the device is inserted into the disc space in a direction so that the wide dimension of the device is substantially parallel to the body of the vertebrae. The device is then rotated so that the wide dimension of the device becomes perpendicular to the vertebral body so as to cause the disc space height to be restored. Bone graft material is then introduced through a fill tube coupled to the device so that the bone graft material is distributed on both sides of the center plate and into the disc space.
Owner:THOMPSON MIS
Multi-density polymeric interbody spacer
InactiveUS20110015743A1High strengthEnhance positioning and fitSpinal implantsCoatingsPorosityUltimate tensile strength
A multi-density polymeric interbody spacer formed from biocompatible material for osteoconductivity includes multiple density regions of different porosity to provide both strength and osteoconductivity. An interface region is formed between the density regions to provide both direct adhesion and mechanical interlocking between the different density regions to increase the strength of the multi-density polymeric interbody spacer. A method for forming the multi-density polymeric interbody spacer includes curing a first density region to achieve a first target porosity. A second density region may then be molded to the first density region to achieve a second target porosity. A portion of the second density region partially flows into pores of the first density region, providing direct adhesion and mechanical interlocking between the first and second density regions.
Owner:DOCTORS RES GROUP
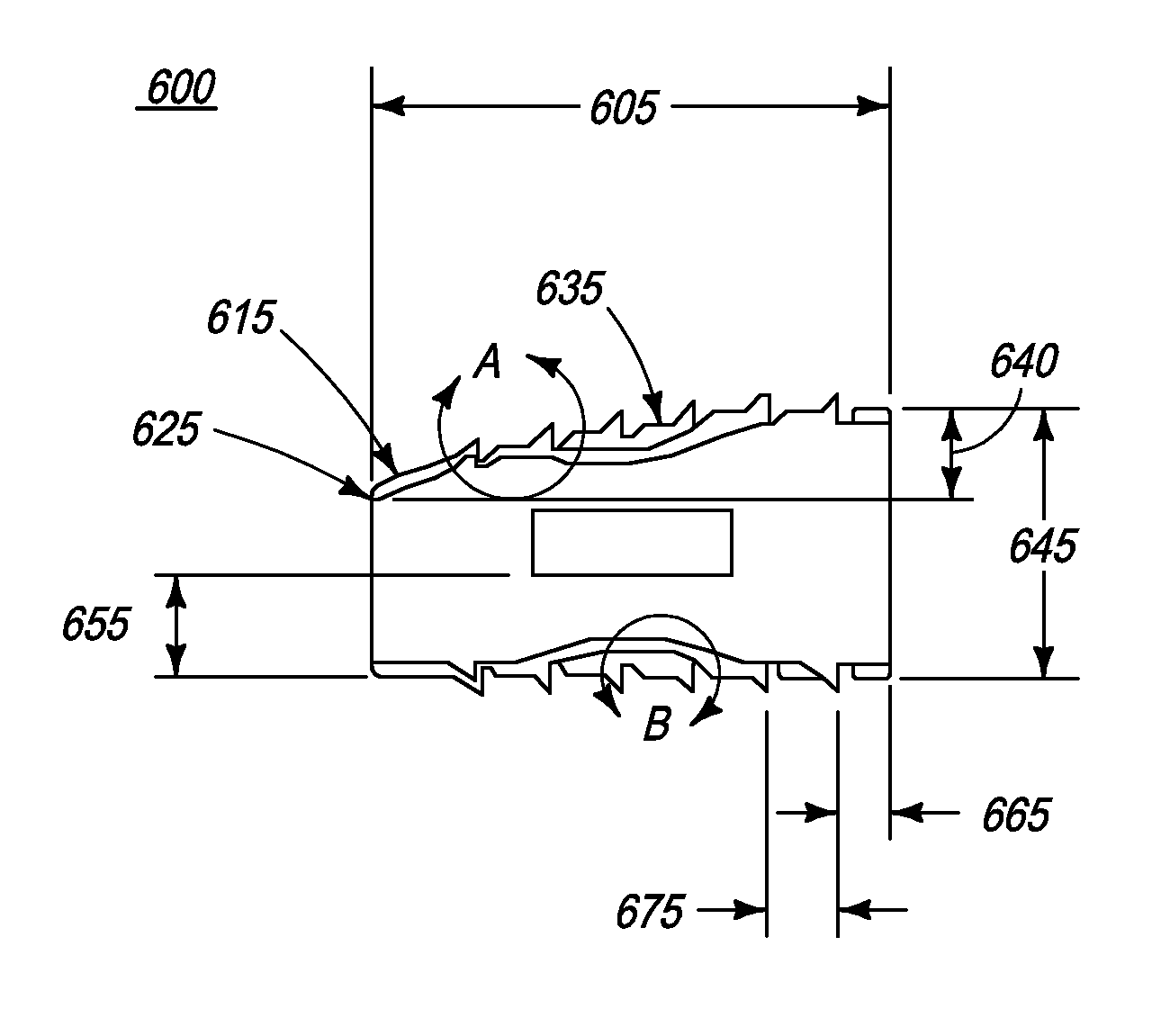
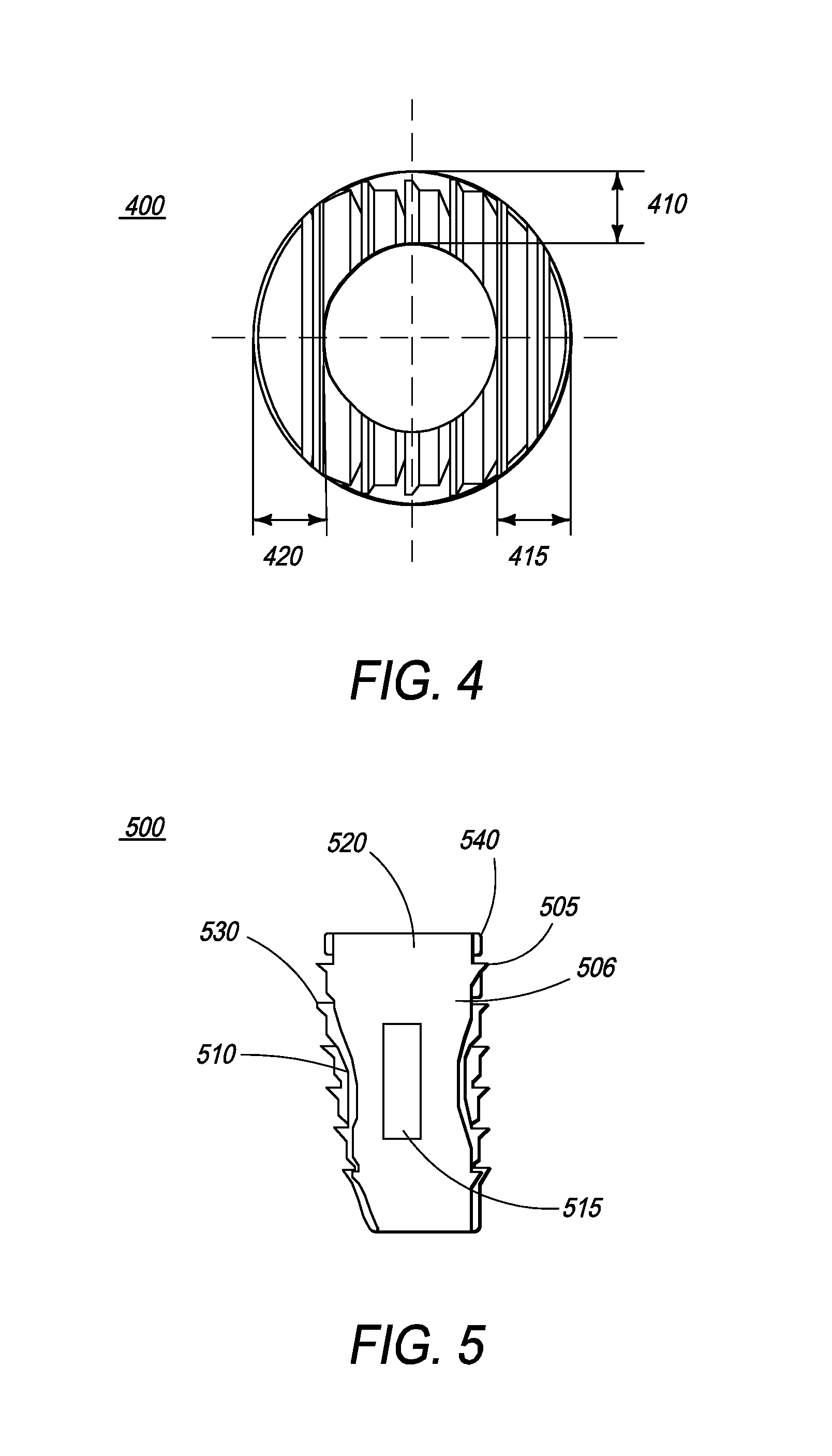
Implantable intervertebral fusion device
InactiveUS20080097610A1Restore heightRecovery heightBone implantSpinal implantsIntervertebral fusionBiomedical engineering
The present invention relates to an implantable intervertebral fusion device for use when surgical fusion of vertebral bodies is indicated. The implant is comprised of bone conforming in size and shape with the end plates of the adjacent vertebrae and has a wedge-shaped profile with a plurality of footings and grooves located on the top and bottom surfaces.
Owner:OSPREY BIOMEDICAL
Apparatus, kit, and method for percutaneous intervertebral disc restoration
ActiveUS20140180415A1Improves Structural IntegrityIncrease expensesJoint implantsSpinal implantsIntervertebral discCatheter
An assembly for replacing a vertebral disc. The assembly includes an implant and a device for positioning the implant between two adjacent vertebrae. The implant includes a body having an internal compartment enclosed by an outer wall, and an endoskeleton coupled to the body. The device includes a cannula, a container coupled to the cannula, the container having a first end and a second end, a plunger threadingly coupled to the cannula and to the first end of the container, and a conduit coupled to the second end of the container and in fluid communication with the body of the implant. The implant is positioned within the cannula in an undeployed state and is deployed within a vertebral disc to receive a biological fluid in a second state.
Owner:PERCHERON SPINE LLC
Implant for alleviating pressure on intervertebral disks and method for restoring the height of and alleviating pressure on an intervertebral space
InactiveUS20080109082A1Easy to carryReduce pressureInternal osteosythesisSpinal implantsSpinal columnIntervertebral space
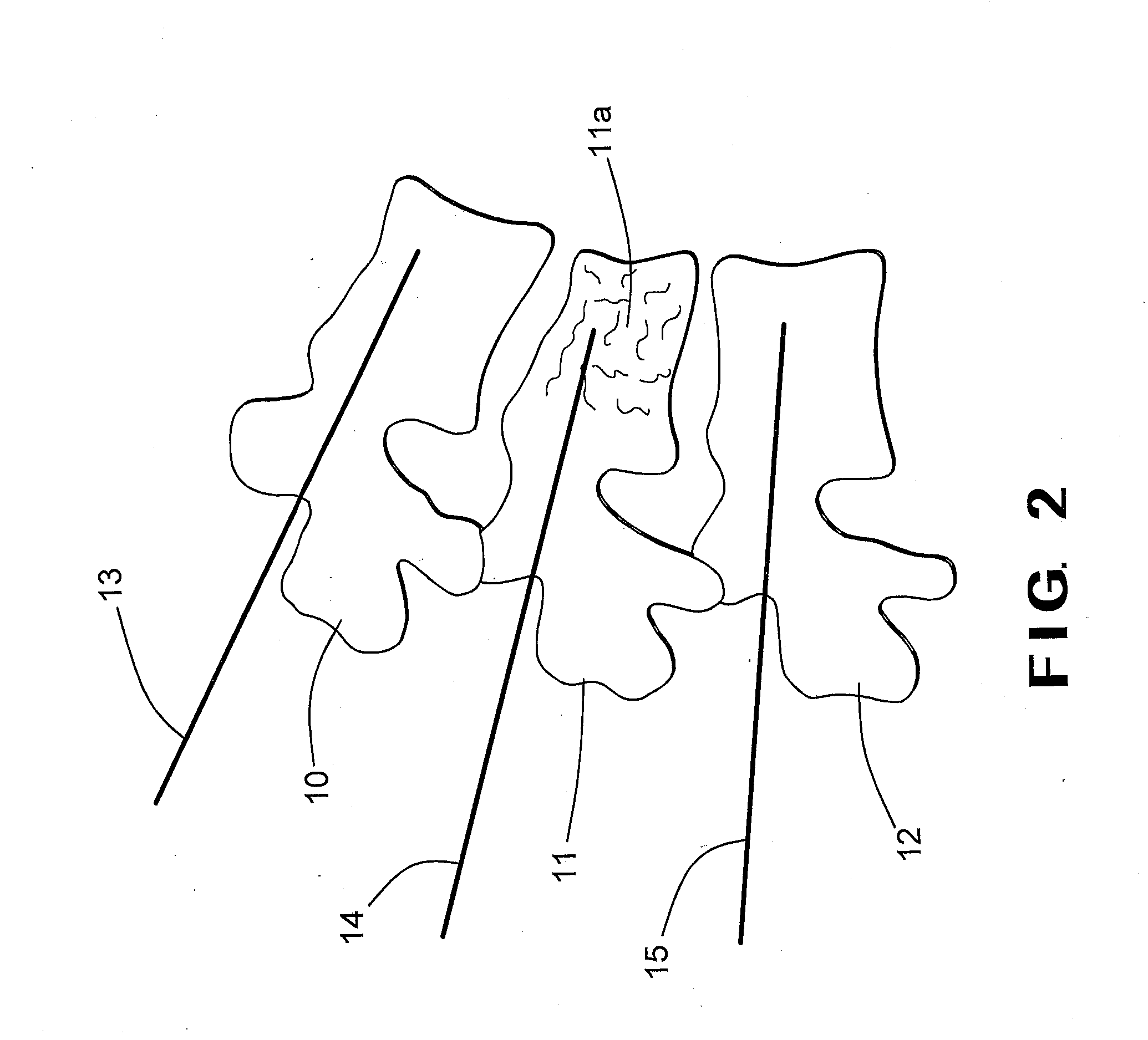
In order to improve an implant (10) for alleviating pressure on intervertebral disks, for restoring the height of and alleviating pressure on an intervertebral space of a human or animal spinal column, comprising at least two bearing elements (24, 26) for a spinous process (14, 16) each for abutting and / or securing the implant on one or two spinous processes of adjacent vertebra of the spinal column, such that as far as possible only one single operation is required to restore the height of and alleviate pressure on the intervertebral space it is suggested that the implant (10) be produced from a biocompatible, resorbable material. Furthermore, a method for restoring the height of and alleviating pressure on an intervertebral space of a human or animal spinal column is suggested.
Owner:AESCULAP AG
Implantable intervertebral fusion device
InactiveUS8043377B2Recovery heightBone implantSpinal implantsIntervertebral fusionBiomedical engineering
Owner:OSPREY BIOMEDICAL
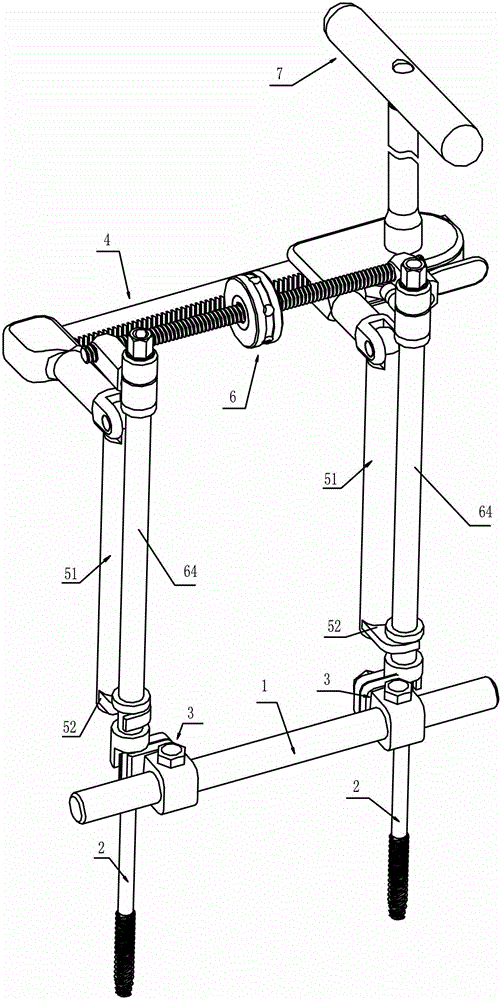
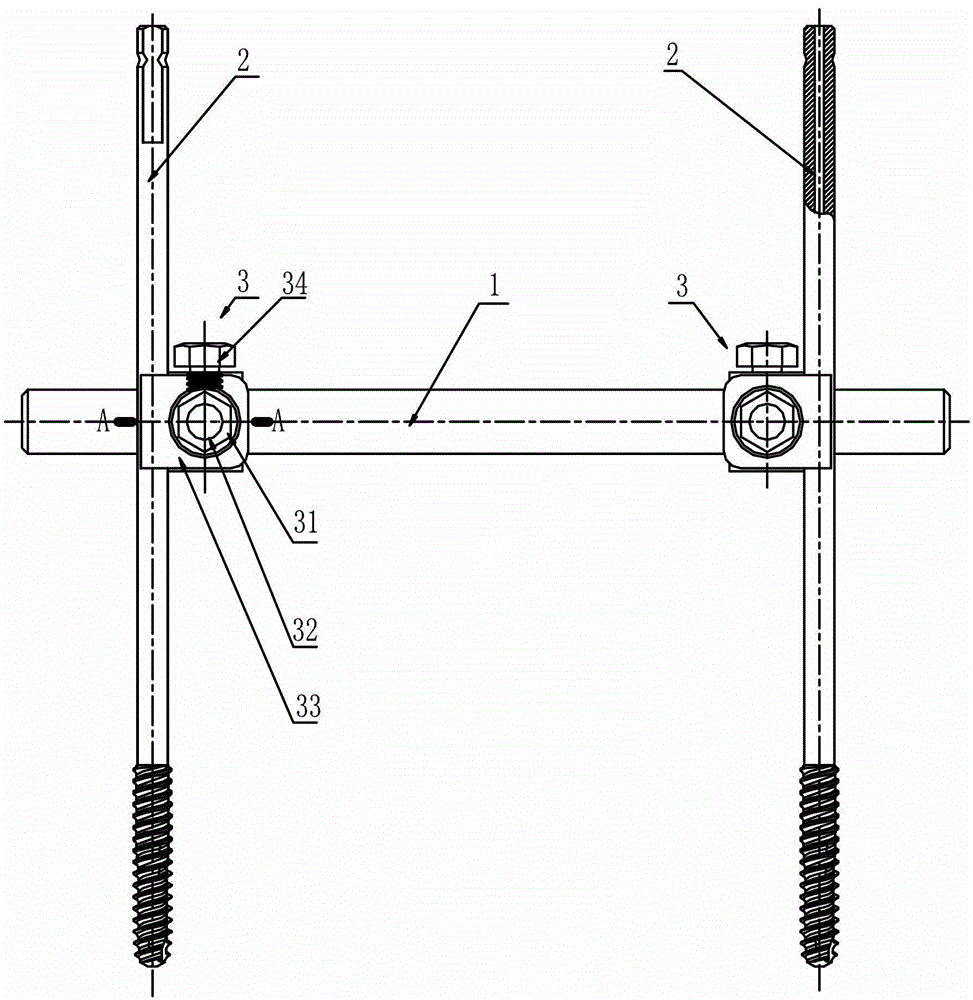
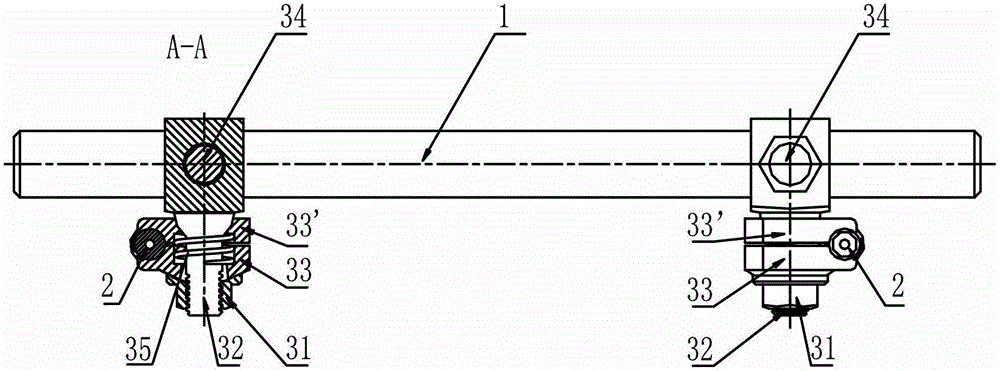
External thoracolumbar vertebra distraction repositor
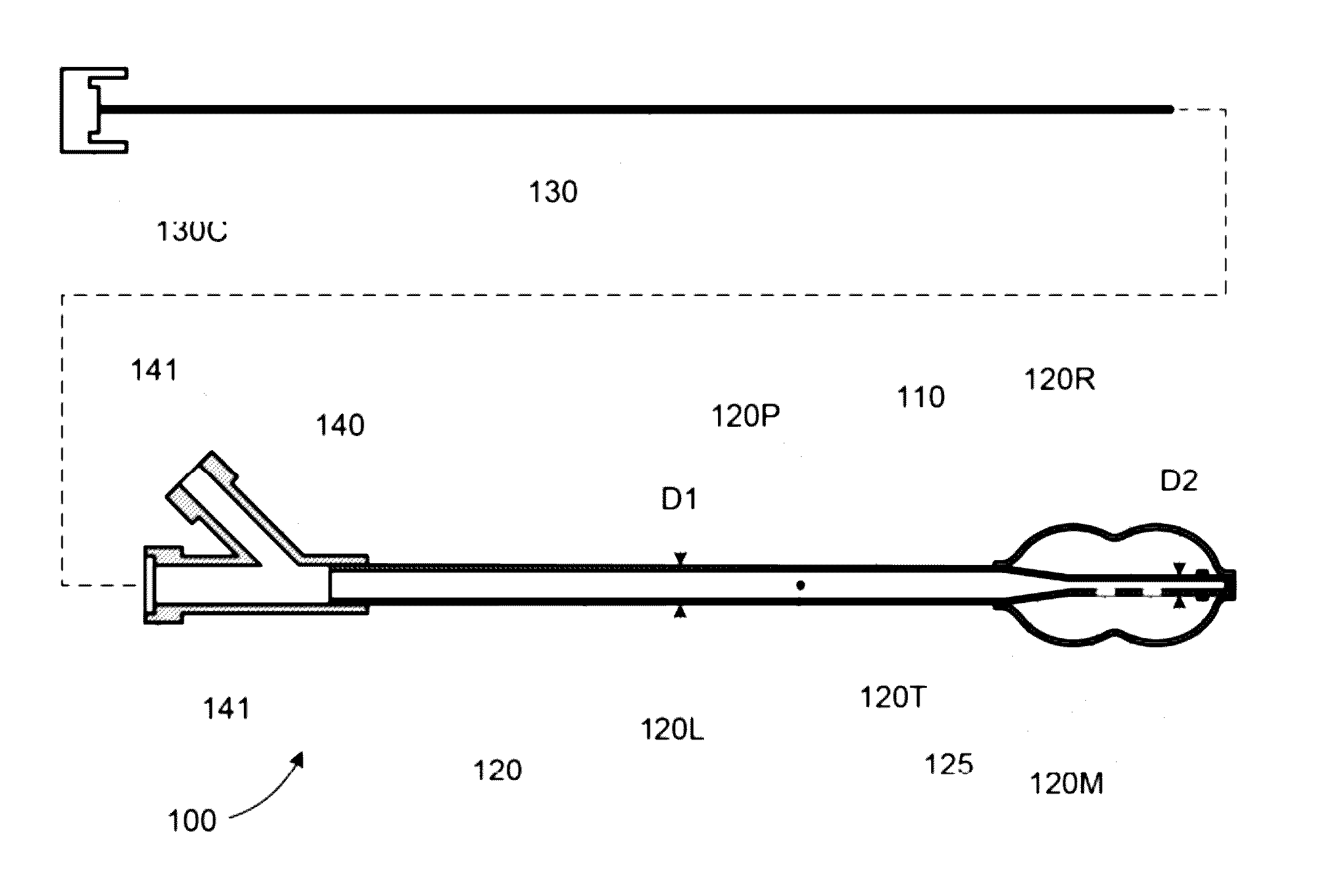
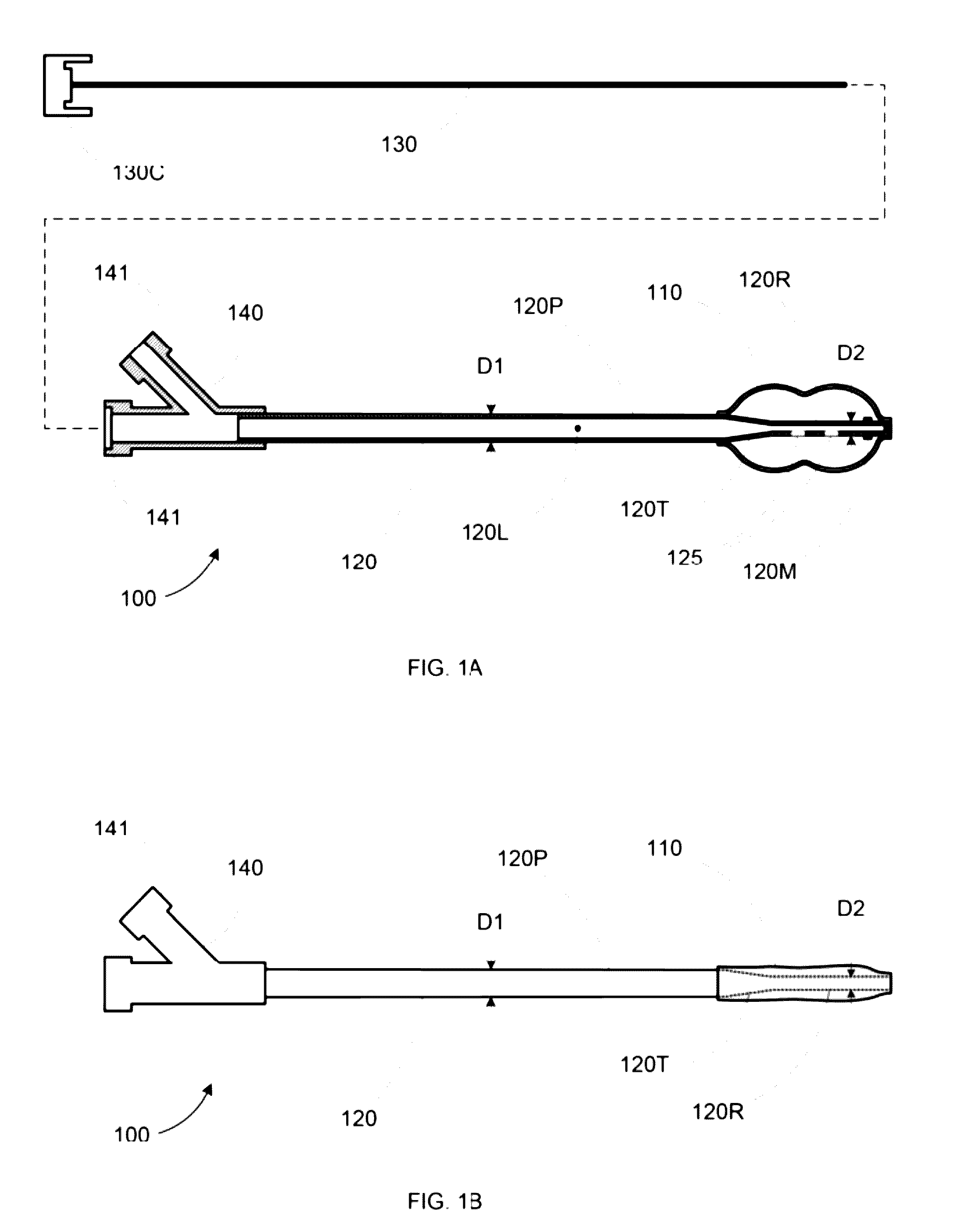
The invention discloses an external thoracolumbar vertebra distraction repositor which comprises a fixed support, two hollow screws, a distracter, a pressurizer and a T-shaped socket spanner; the fixed support comprises a beam and two sets of clamps; the distracter comprises a body and two distraction rods, and one end of the distraction rod is provided with a C-shaped foot hook which is vertical to the distraction rod; the pressurizer comprises a thread rod, a connector and an extension rod; the connector comprises a nut which is connected with the thread rod and a lantern ring which is sleeved on the extension rod, and the lantern ring and the extension rod as well as the lantern ring and the nut are respectively connected with each other rotatably in two intersected planes. The distraction repository can spread the vertebral body to the maximum height according to the practical conditions, and through utilizing the self restrictive tensile forces of the anterior and the posterior longitudinal ligaments of the vertebral body, the vertebral body can be effectively corrected to be backwards protruded for an angle of 2-8 degrees; and moreover, the heights of the anterior, the center and the posteriors can also be effectively reposed. In addition, the distraction repository is also suitable for spreading, restoring and reposing the burst fractures without symptoms of stressed spinal cord and neurological deficit when vertebral body paries posterior fracture is burst in the spinal canal.
Owner:冯其金 +2
Anterior inflation balloon
ActiveUS20110106184A1Great distal expansionRaise the possibilityStentsBalloon catheterSurgical departmentVertebra
An inflatable bone tamp for performing a minimally invasive surgical procedure includes an inflatable structure that exhibits an outwardly tapering expansion profile. The outward taper can beneficially allow the inflatable bone tamp to exert greater targeted force in difficult environments. For example, in a vertebra with a compression fracture, the outwardly tapering expansion profile of the inflatable bone tamp can result in greater lifting force being applied to the endplates of the vertebra, thereby increasing the likelihood of restoring the height lost due to the compression fracture.
Owner:KYPHON
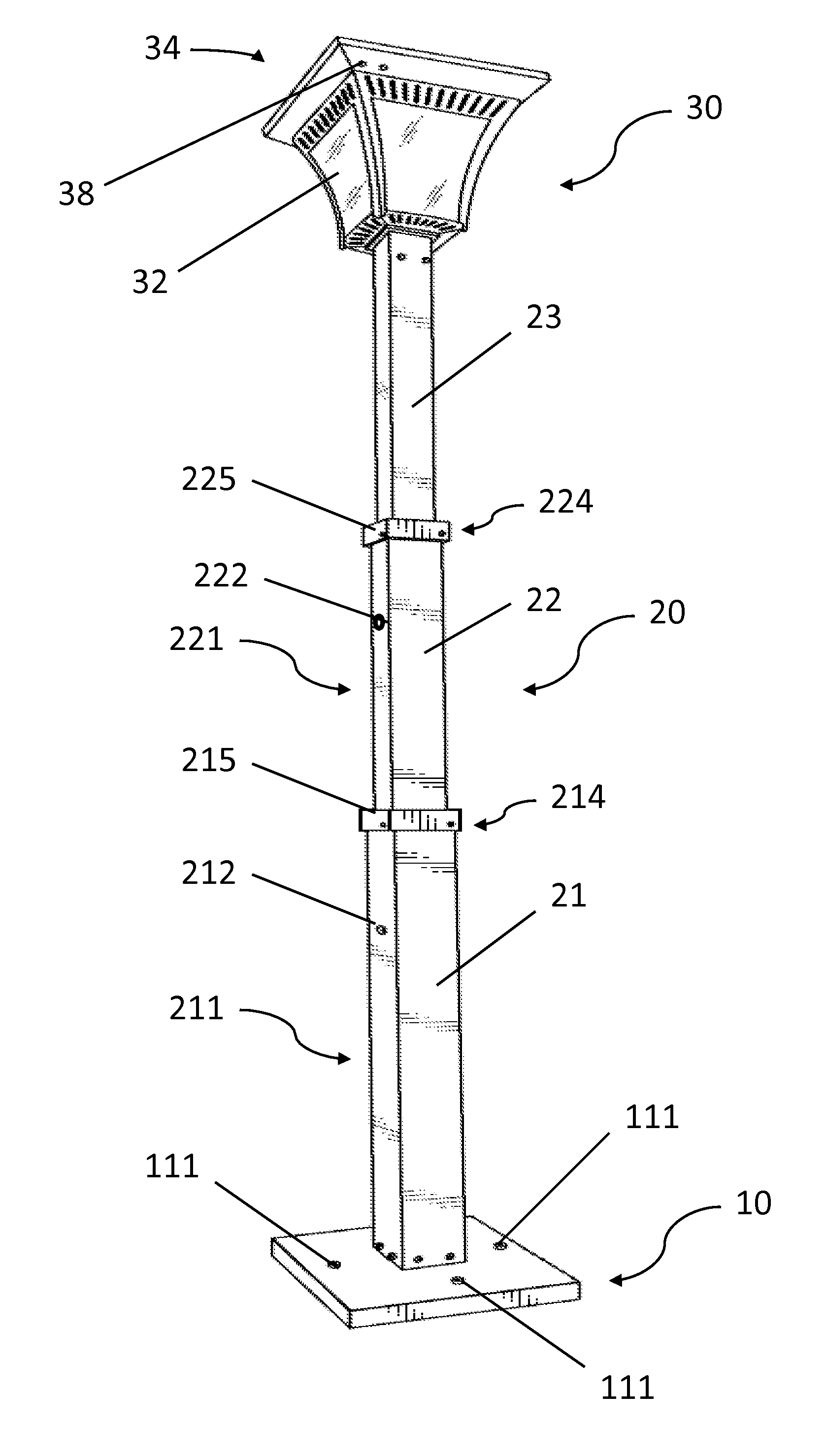
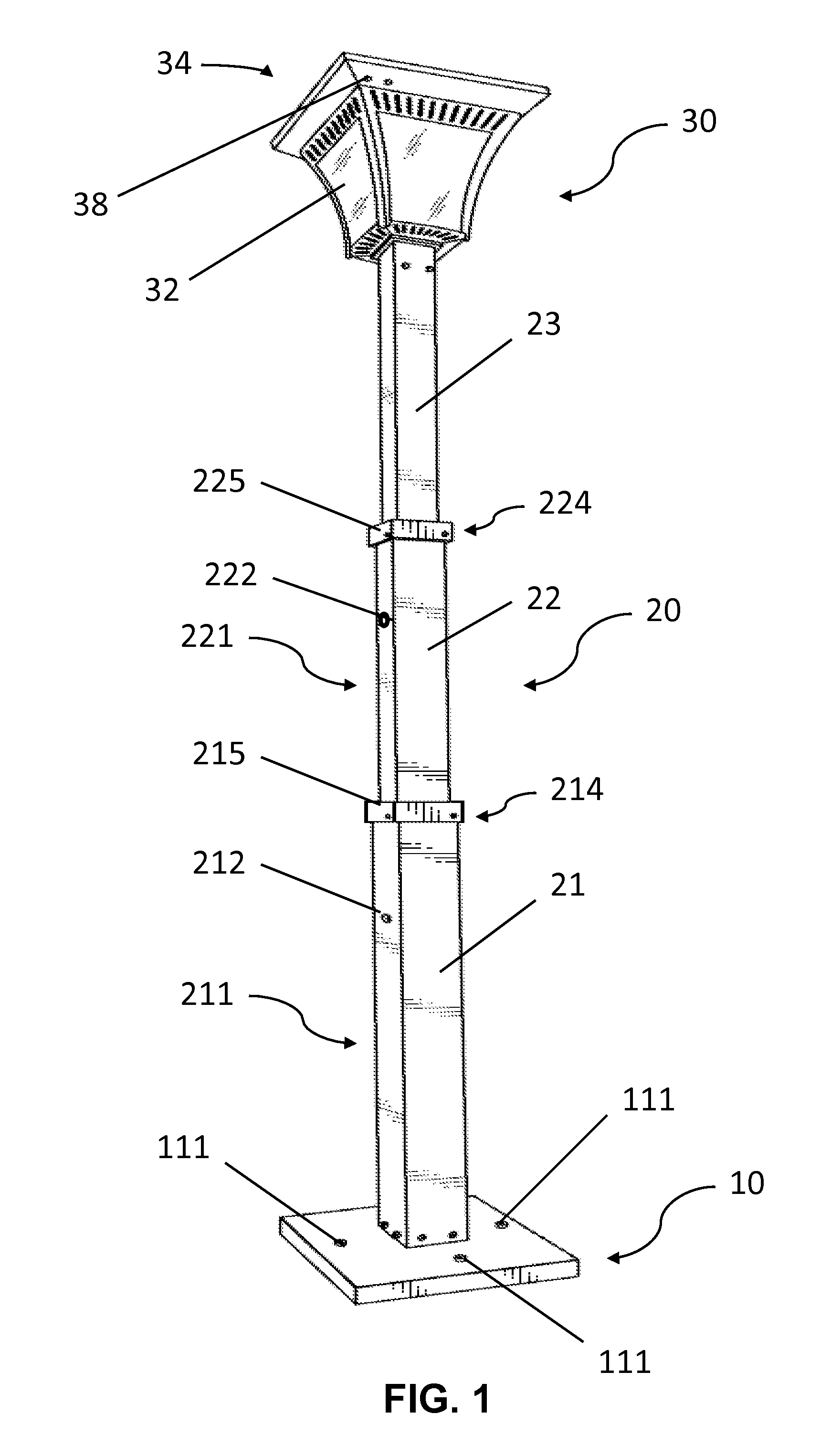
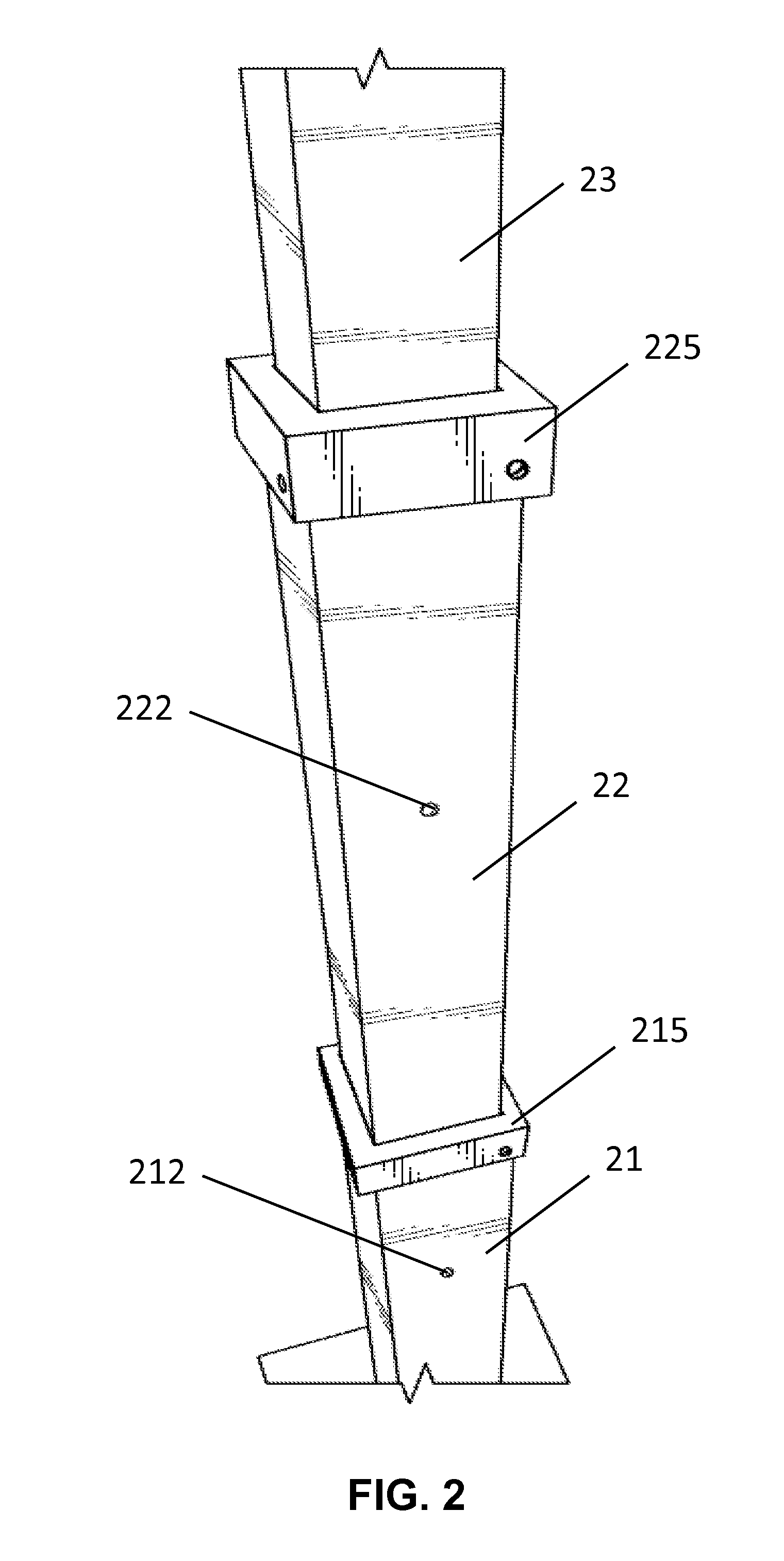
Solar lamp with adjustable height
InactiveUS20150103518A1Easily and quickly and uninstalledEasy to adjustMechanical apparatusLighting support devicesRechargeable cellEffect light
A solar lamp may include a bottom plate, a lamp post, and a lighting complex. In one embodiment, the lamp post is fixed to the bottom plate and has three segments. The three segments are retractable thereby allowing the height of the lamp post to be adjusted. The lighting complex is installed at the top end of the lamp post and comprises a housing and a solar panel which is placed onto the top side of the housing. The housing may include a frame and with glass panels installed, and enclose a LED lamp, a rechargeable battery, and required wirings. The present invention is advantageous because the solar lamp can be easily and quickly installed or uninstalled, and the height of the lamp post can be easily and conveniently adjusted.
Owner:IP POWER HLDG
Interverterbral fusion implement
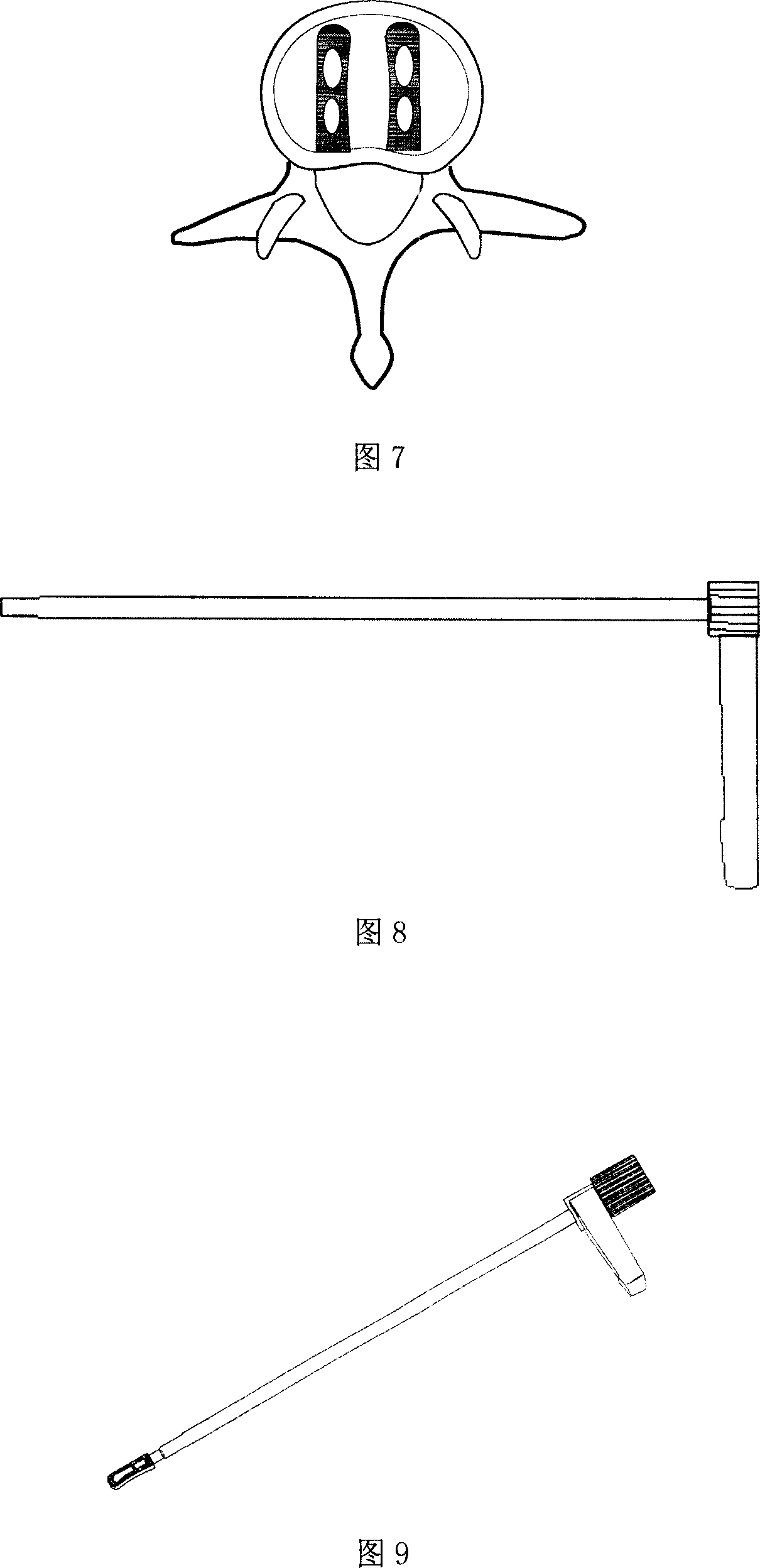
InactiveCN1436518ANo foreign body reactionPromote bone fusionInternal osteosythesisProsthesisHuman bodyBiocompatibility Testing
The interverterbral fusion implement for cervical vertebra anterior operation is one hollow column with polygonal or circular cross section, holes on side wall and sharp teeth on the upper and the lower surfaces. It is made of polymer material capable of being absorbed by human body, poly(DL-lactic acid), poly(L-lactic acid), polyglycolic acid or their copolymer. It has determined early-stage locking effect, late-stage bone fusion effect, good biocompatibility and proper mechanical structure, and may be self-degraded after being fused with vertebra. It results in no rejection and may be also used in thoracic vertebra and lumbar vertebra operation.
Owner:成都迪康中科生物医学材料有限公司
Spinal column dynamic connection rod
InactiveCN102106750AExcellent structureImprove performanceInternal osteosythesisSpinal columnPhysiological movement
The invention provides a spinal column dynamic connection rod, comprising an upper connection part, a lower connection part and an elastic piece, wherein the upper connection part and the lower connection part are connected with each other via the elastic piece. The spinal column dynamic connection rod is characterized in that: the upper connection part, the lower connection part and the elastic piece are integrally formed; the elastic piece is provided with an elastic hole; and an elastic groove is formed on an external periphery of the elastic piece. The spinal column dynamic connection rodprovided by the invention can realize stability of the spinal column and keep the normal physiological movement of the spinal column with a simple structure.
Owner:SHANGHAI MICROPORT ORTHOPEDICS
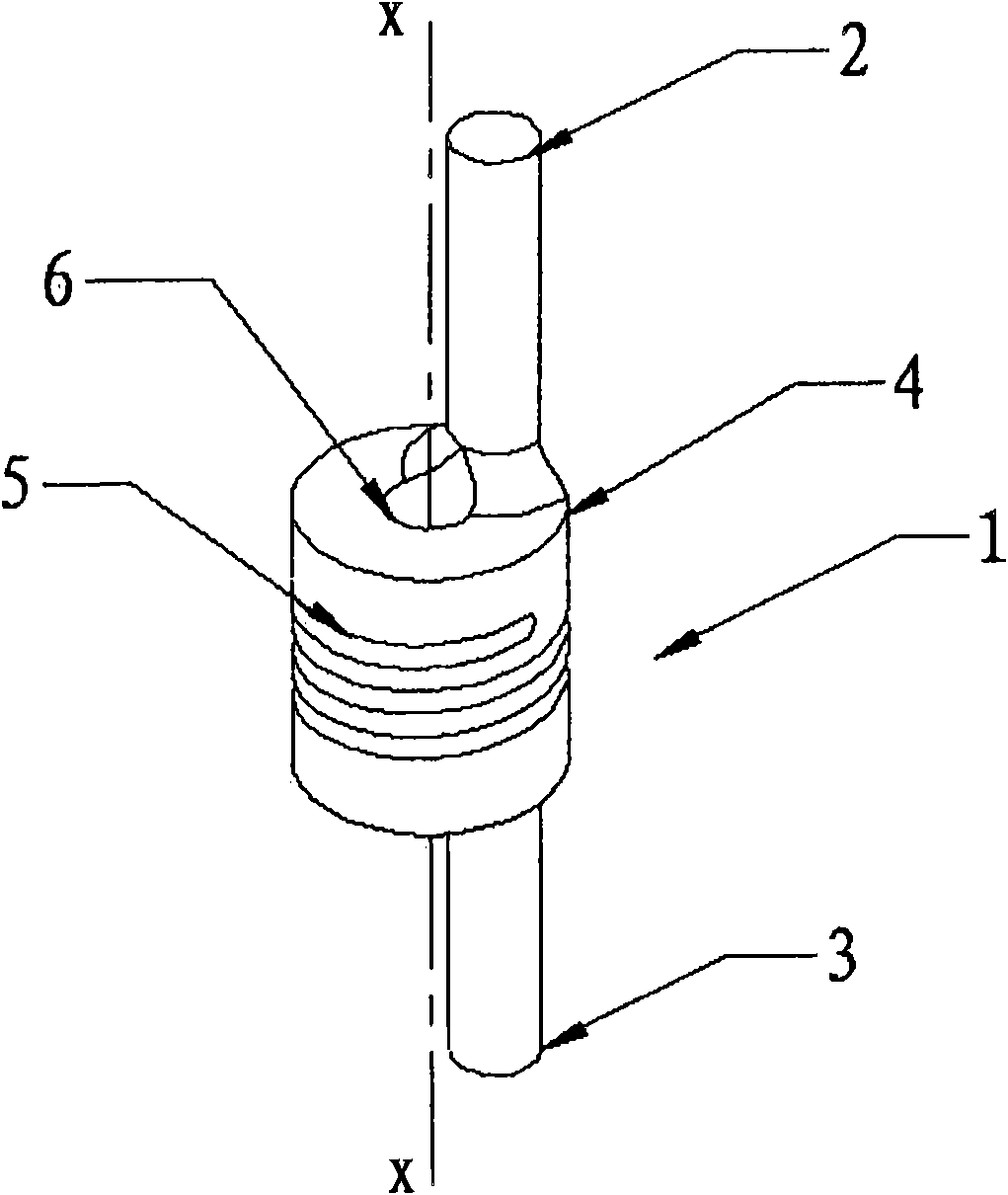
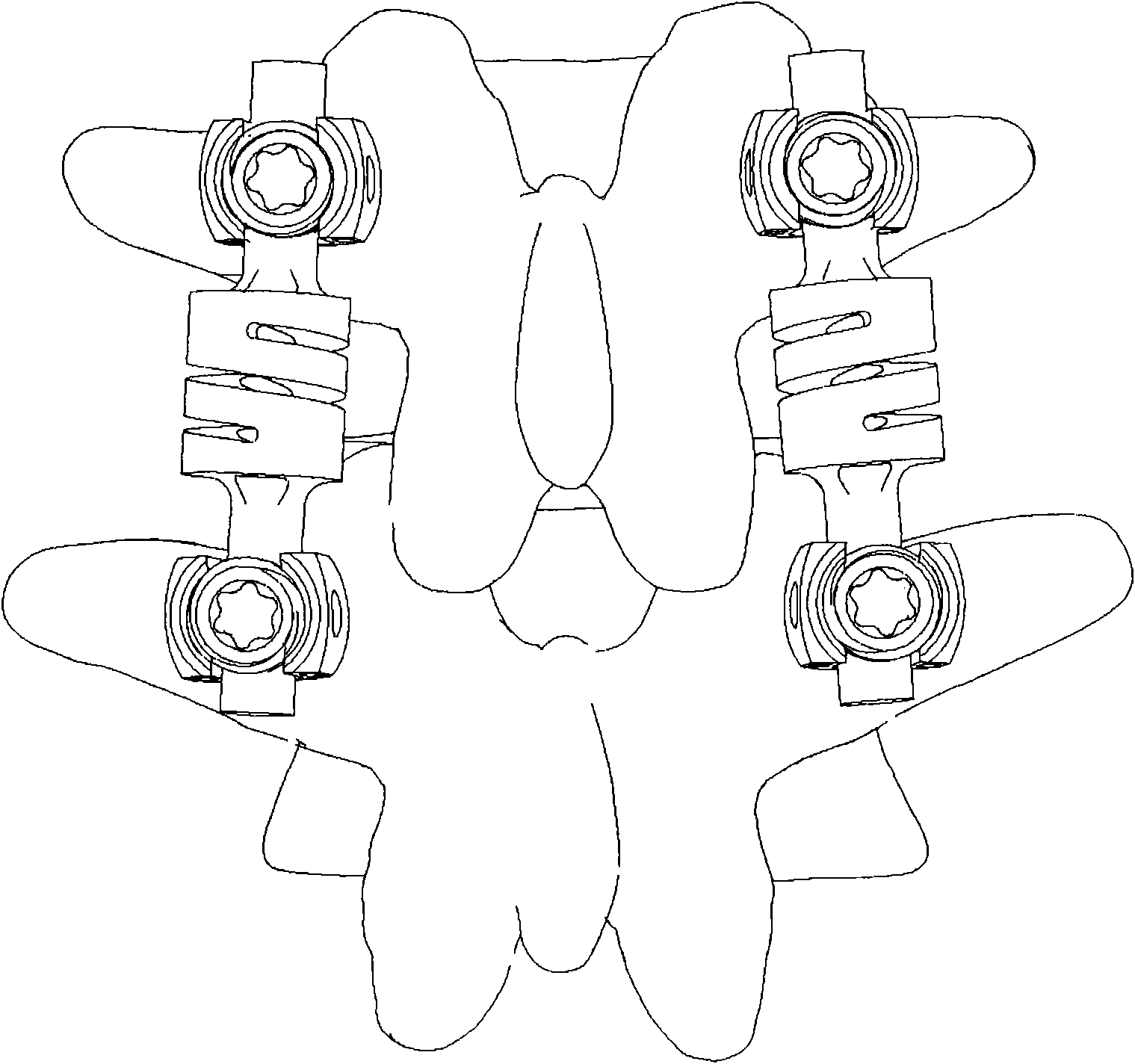
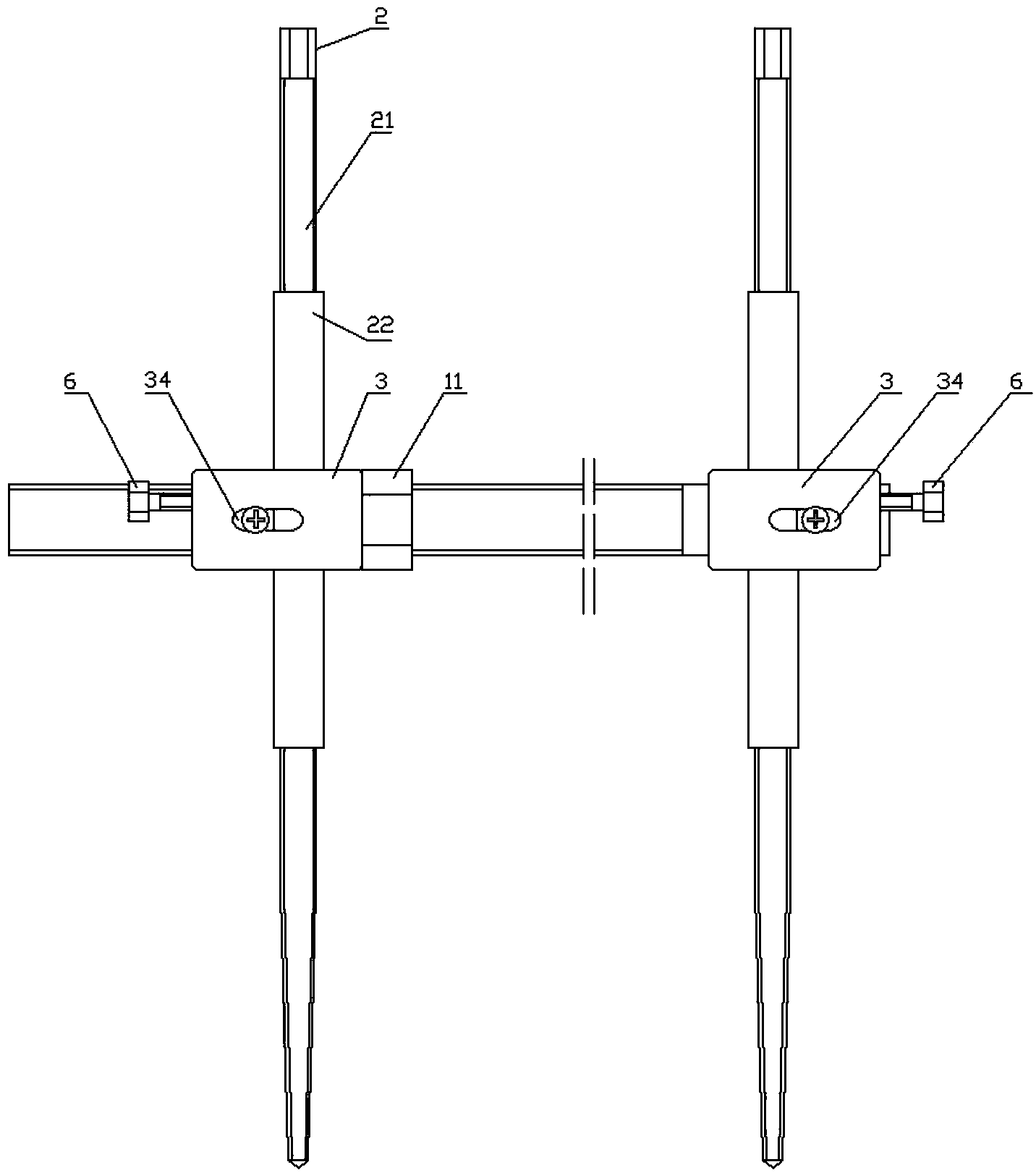
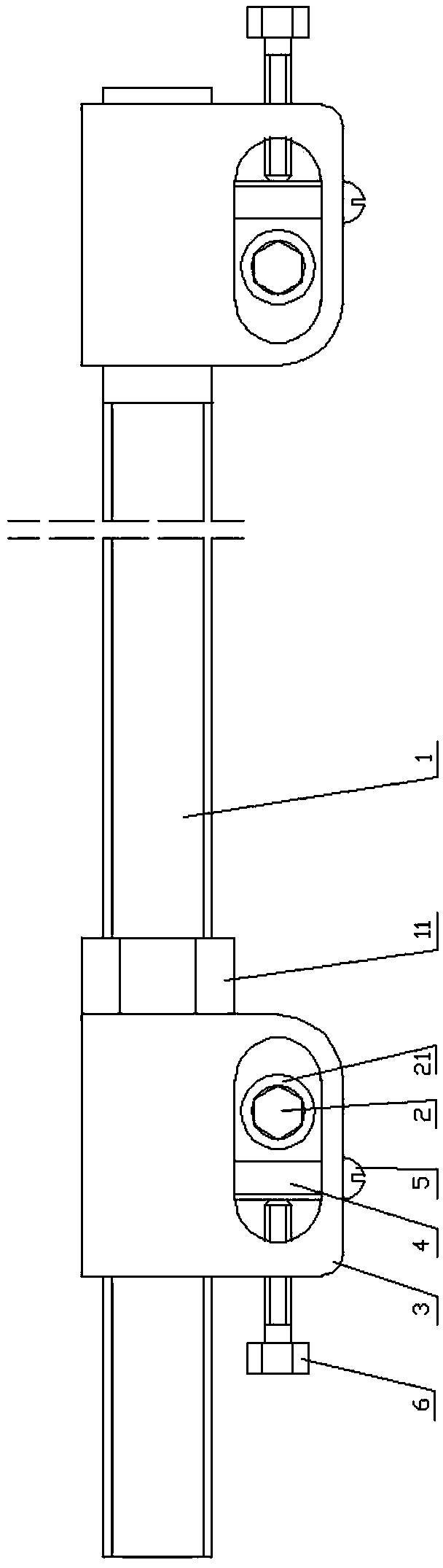
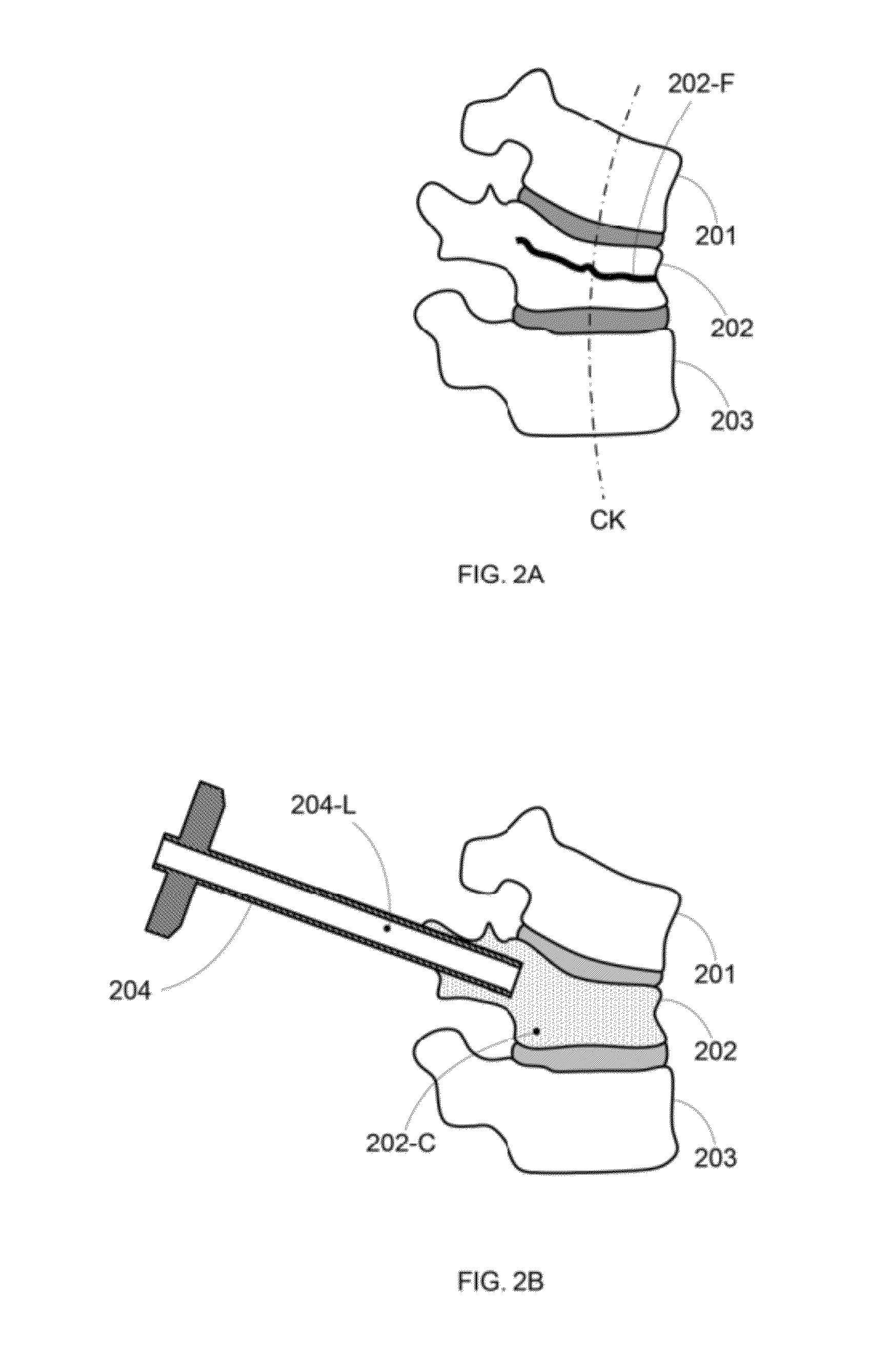
External fixing device for minimally invasive spine leverage reduction
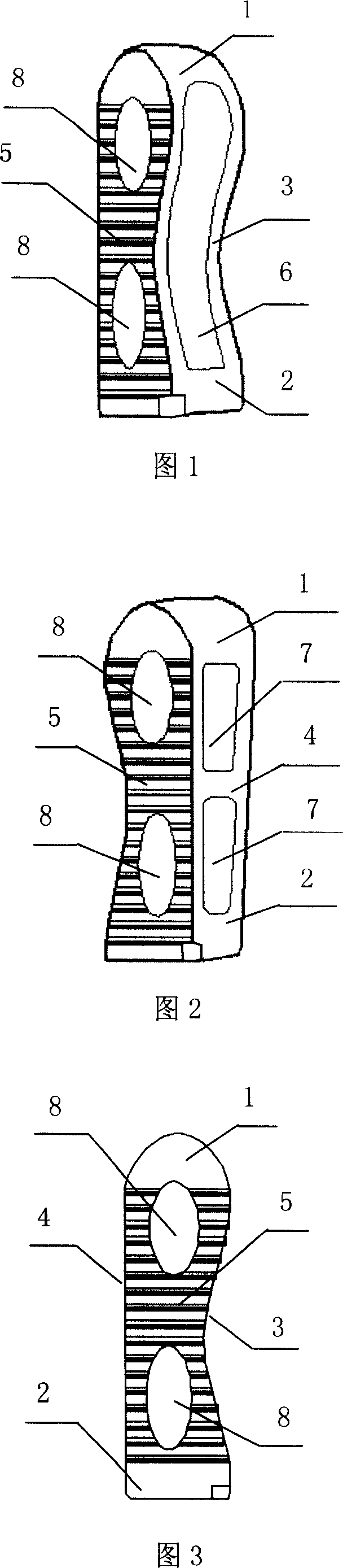
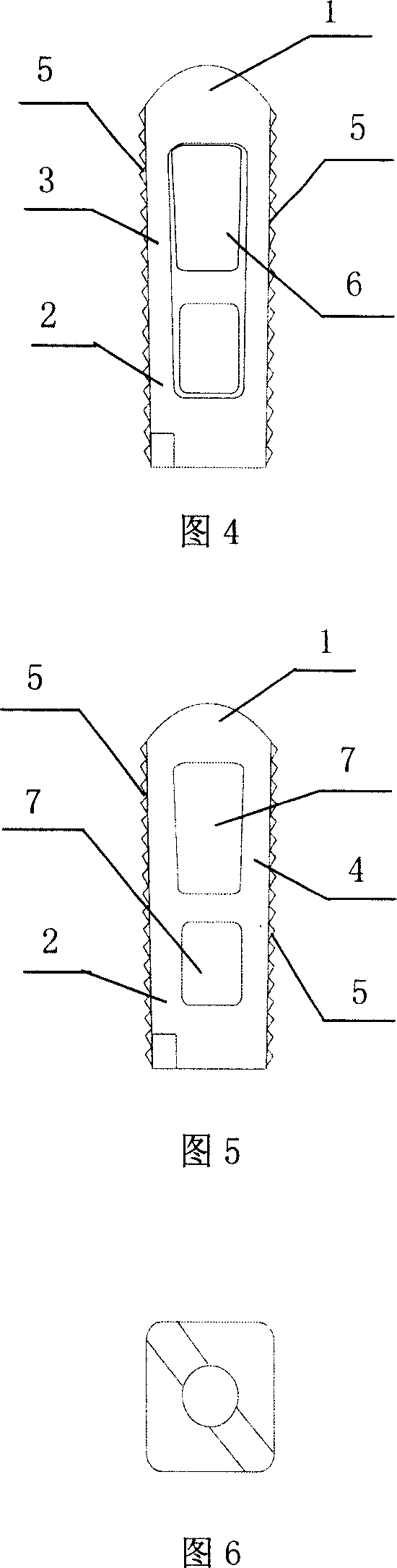
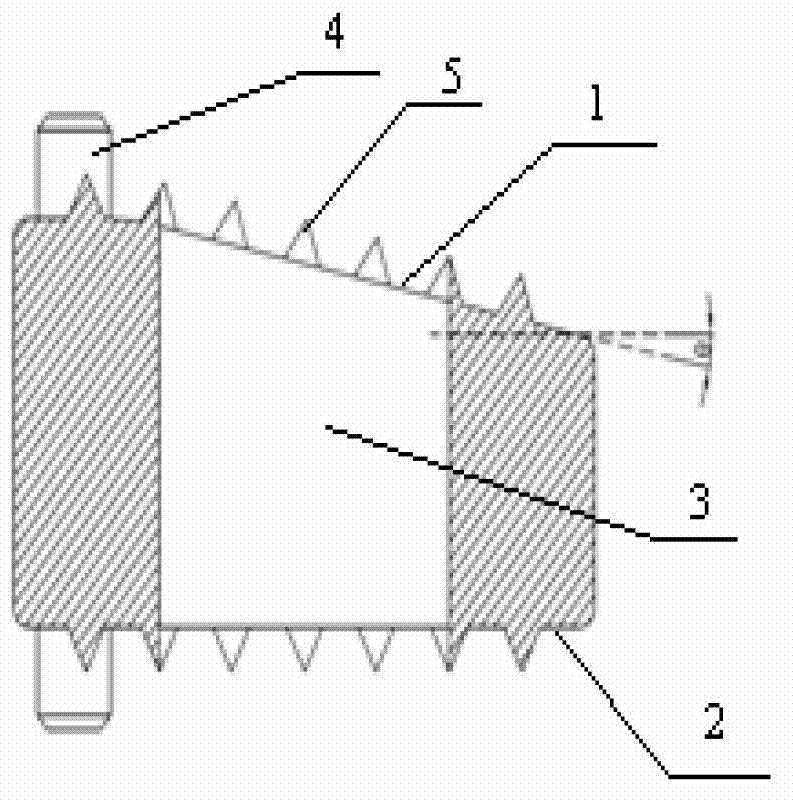
InactiveCN103519874ATraditional open surgery is riskyRecovery heightExternal osteosynthesisLUMBAR SPINE FRACTURELumbar vertebrae
An external fixing device for minimally invasive spine leverage reduction comprises a main screw rod (1), leverage percutaneous pedicle screws (2) and leverage devices, wherein the leverage devices comprise connecting pieces (3), pushing sheets (4), fixing screws (5) and leverage screws (6). The left end of the main screw rod (1) is movably arranged in a main screw rod hole (31) of the left leverage device and is located through a nut (11), the right end of the main screw rod (1) is fixedly arranged in a main screw rod hole (31) of the right leverage device, and the left leverage device and the right leverage device are identical in structure and are opposite in direction. The leverage percutaneous pedicle screws (2) are arranged in leverage percutaneous pedicle screw holes (33) of the leverage devices through anti-abrasive cannulas (22). According to the external fixing device for the minimally invasive spine leverage reduction, reduction of thoracic waist fractures can be achieved through leverage opening by operating outside the body, skin soft tissue does not need to be cut open, a patient can be off the bed in one to three days after the external fixing device for the minimally invasive spine leverage reduction is relieved, the patient can recover to a movement state before the injury in a short period of time, and the external fixing device for the minimally invasive spine leverage reduction is especially suitable for 60-100-year-old thoracic waist fracture patients.
Owner:宋西正 +1
Inflatable bone TAMP with predetermined extensibility
ActiveUS20120197321A1Improve abilitiesRestore heightSurgical needlesJoint implantsElastomerExtensibility
An inflatable bone tamp for performing a minimally invasive surgical procedure includes an inner shaft that incorporates a longitudinally extensible section. The predetermined longitudinally extensible section permits greater control over the elongation of the inflatable portion of the inflatable bone tamp, thereby allowing performance characteristics to be tailored to specific procedures and / or situations. The predetermined longitudinally extensible section can be implemented in various ways, such as a spring, elastomer, or mechanical linkage, among others.
Owner:KYPHON
Minimally Invasive lumbar fusion device and its use method
InactiveCN101015477AEasy to insertReduce human injuryInternal osteosythesisSpinal implantsVertebraLumbar
The invention discloses a micro-injury intervertebral fusing tool and using method, which is characterized by the following: the surface between fusing tool head and rear possesses two end-plate contact faces, internal plug face and external plug face; the head of fusing tool is outward arc to minimize from back to front, which transmits between internal and external plug sides. The using method comprises the following steps: (a) plugging internal and external plug sides into intervertebral along level direction; (b) rotating fusing tool by 90 deg along axle to erect the fusing tool; contacting end-plate contact surface and vertebra end-plate tightly; realizing micro-injury operation effectively.
Owner:王建华
Medical degradable magnesium alloy interbody fusion cage
InactiveCN102784415AAvoid non-specific inflammationImprove stabilityInternal osteosythesisSpinal implantsSpinal cageStress shielding
The invention discloses a medical degradable magnesium alloy interbody fusion cage, relates to an interbody fusion cage and aims at solving problems that inflammation is caused due to acidic materials which are produced by existing interbody fusion cages during degradation and stress shielding can occur due to inappropriate selection of elasticity modulus. The medical degradable magnesium alloy interbody fusion cage is made of Mg-Sn-Mn series alloy or Mg-Sn-Mn-Zn series alloy; the medical degradable magnesium alloy interbody fusion cage comprises an upper end plate contact surface (1) and a lower end plate contact surface (2), a through hole (3) is opened at the center of the fusion cage, the upper end plate contact surface (1), the lower end plate contact surface (2) and the through hole (3) are polygons which are of the same shape, two protuberant studs (4) are respectively arranged on the upper end plate contact surface (1) and the lower end plate contact surface (2), and the upper end plate contact surface (1) can be an inclined surface to form an angle theta with the lower end plate contact surface (2). The medical degradable magnesium alloy interbody fusion cage can be applied to the field of degradable and absorptive medical instrument manufacturing.
Owner:HARBIN ENG UNIV
Anterior inflation balloon
ActiveUS8262609B2Great distal expansionRaise the possibilityStentsBalloon catheterSurgical departmentSurgical procedures
Owner:KYPHON
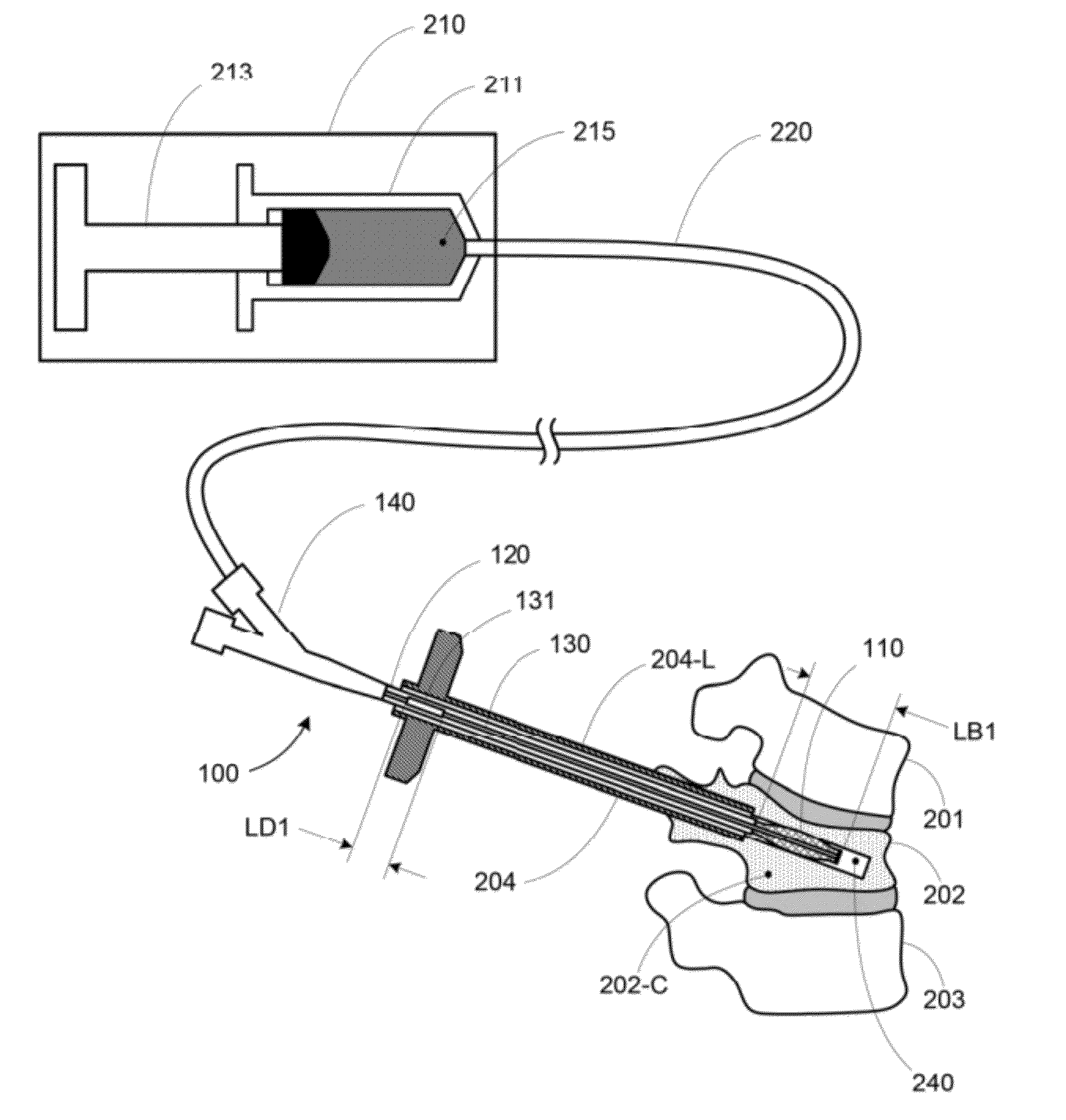
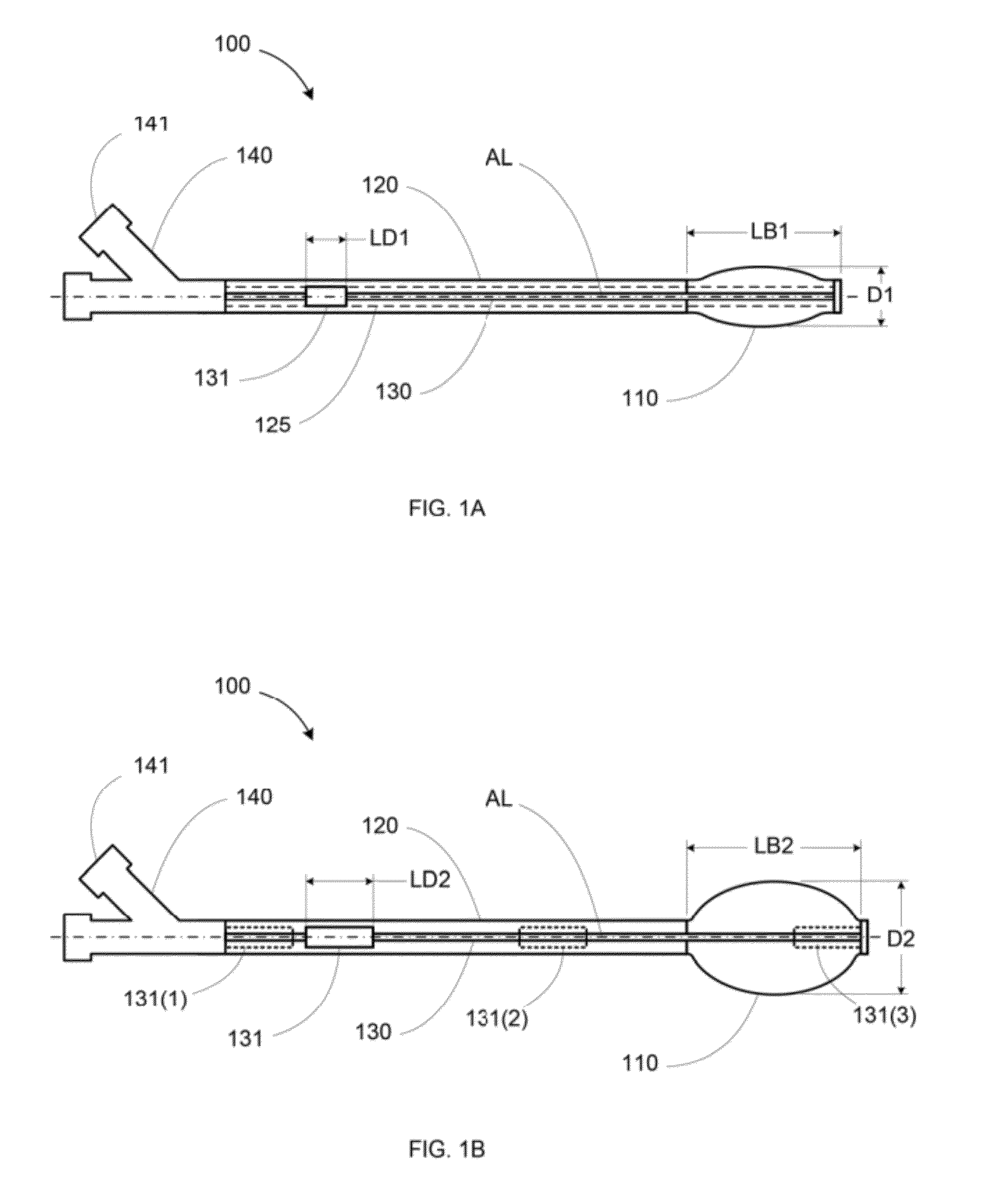
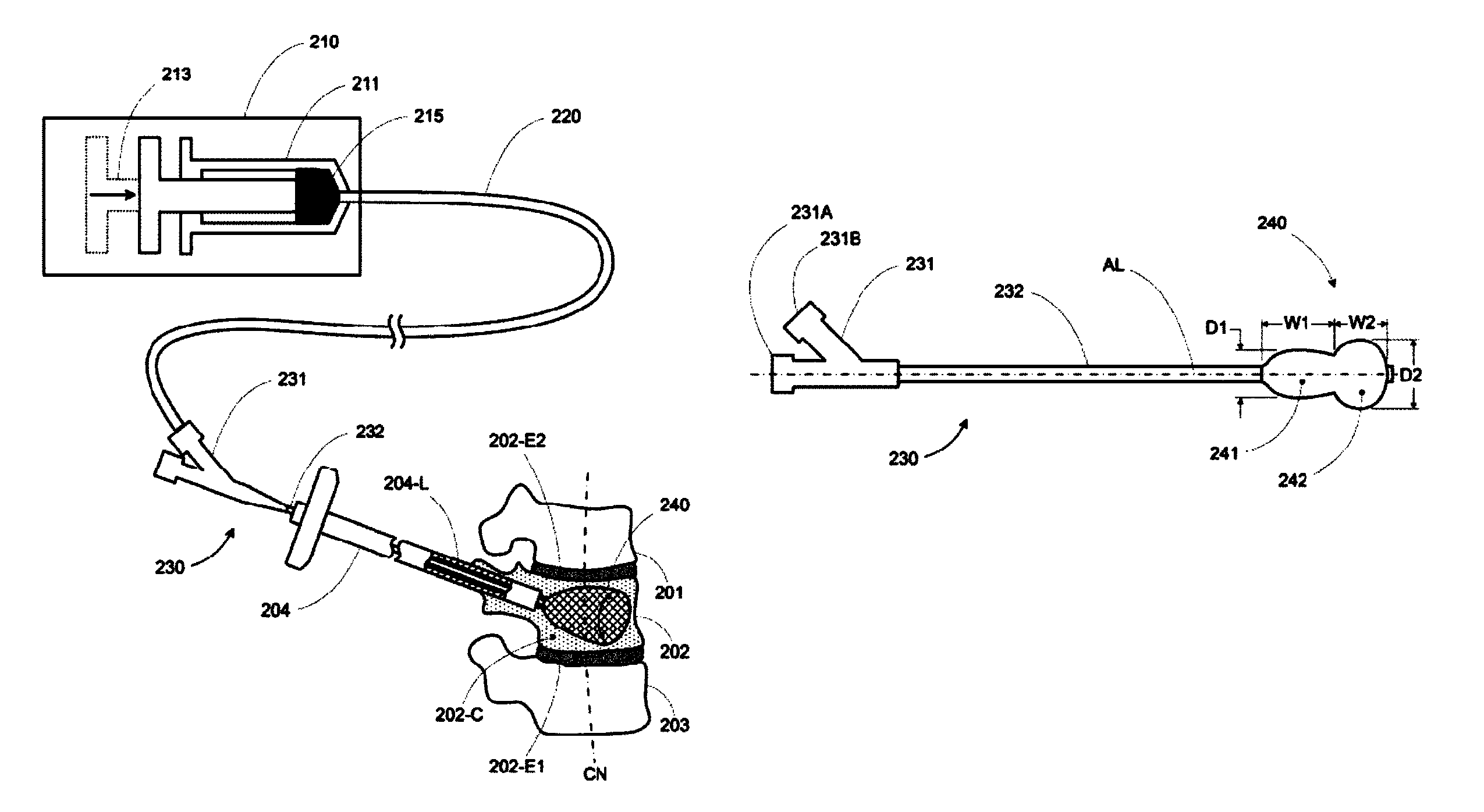
Vertebral body expansion balloon catheter
PendingCN106422038ARecovery heightAchieve targeted expansionBalloon catheterGuide wiresBalloon catheterGuide tube
The invention discloses a vertebral body expansion balloon catheter comprising a balloon, an outer tube, an inner tube and a support guide wire; a near-end of the balloon is fixed on an outer wall of the outer tube; the outer tube is communicated with the balloon and pressurizes for the balloon; the inner tube is installed inside the outer tube in a penetrating mode; the inner tube comprises a straight section and a far-end flexible bending section; the flexible bending section extends out from the outer tube and is fixedly connected with the far-end of the balloon; the support guide wire is slidingly contained inside the inner tube; the flexible bending section is kept straight when the support guide wire is inserted and returns bent when the support guide wire is extracted. The flexible balloon structure can effectively support a vertebral body cavity by expanding the balloon, and vertebral body height can be well restored by inserting one-side balloon conduit. Besides, the continuous control of the degree of curvature of the balloon can be achieved by controlling the degree of extraction of the support guide wire from the flexible bending section and associated control devices.
Owner:SHANGHAI KINETIC MEDICAL
Bone stretching dental implant
ActiveCN102038556ARecovery heightEasy to removeDental implantsProsthesisBiomedical engineeringDental implant
The invention relates to the field of an oral cavity medical appliance and discloses a bone stretching dental implant. The bone stretching dental implant is characterized by comprising a fixed sieve and a conveying sieve, wherein the conveying sieve and the fixed sieve are respectively provided with a cylindrical cavity provided with an internal thread along the center longitudinal axis, the cavity contains a coupling screw and a fixed screw or stretching screw, the height ratio of the fixed sieve to the conveying sieve is (1:4)-(1:3), the diameter of the cylindrical cavity of the fixed sieveis smaller than that of the cylindrical cavity of the conveying sieve, and the bottom of the fixed sieve is of an enclosed structure.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Low cost low profile inflatable bone tamp
ActiveUS9554840B2Great distal expansionRaise the possibilityBlunt dissectorsDiagnostic markersMedicineBalloon catheter
An inflatable bone tamp for performing a minimally invasive surgical procedure includes a shaft having a primary region and a reduced diameter region, and an inflatable structure surrounding at least a portion of the reduced diameter region. The reduced diameter region of the shaft allows the deflated size of the inflatable structure to be minimized, while at the same time eliminating the need for the conventional dual lumen balloon catheter construction.
Owner:KYPHON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com