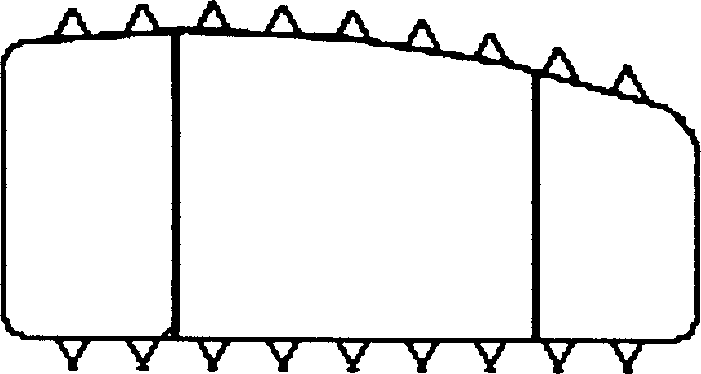
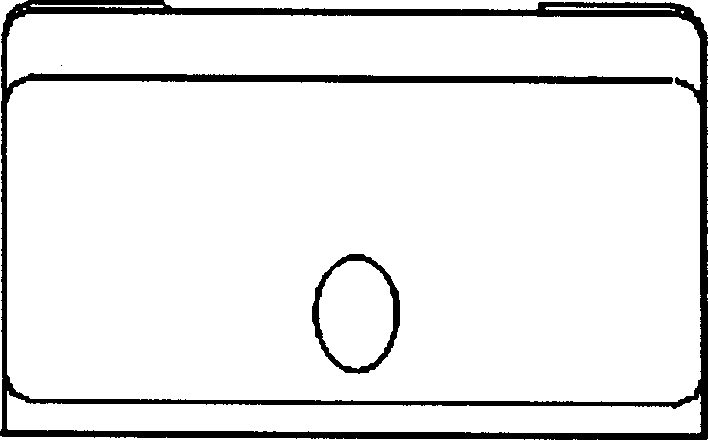
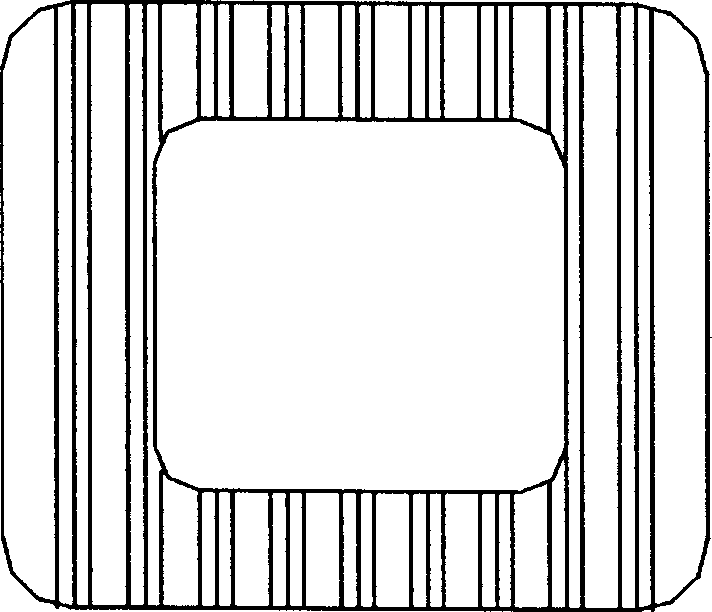
Interverterbral fusion implement
An intervertebral fusion cage and polygon technology, which is applied in the field of medical materials, can solve the problems of foreign body reaction and low incidence of complications, and achieve the effects of no foreign body reaction, excellent curative effect and high fusion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Poly DL-lactic acid material with a molecular weight of 300,000 was molded by an injection machine at an injection temperature of 200° C. to make an intervertebral fusion device. The intervertebral fusion cage is a hollow cylinder with a quadrilateral or hexagonal cross-section. The side wall has a hole with a diameter of 2 mm. The finished product has been tested with a pressure resistance of 913 kg. The in vitro degradation test is shown in Table 1.
Embodiment 2
[0027] Example 2: The copolymer material of DL-lactide and L-lactide (30:70), with a molecular weight of 500,000, was molded by an injection machine to make an intervertebral cage, and the injection temperature was 175°C. The intervertebral fusion cage is a hollow cylinder with an octagonal cross-section and a hole in the side wall with a diameter of 4mm. The finished product has been tested with a pressure resistance of 958 kg. The in vitro degradation test is shown in Table 1.
Embodiment 3
[0028] Example 3: The L-polylactic acid material, with a molecular weight of 400,000, was molded by an injection machine to make an intervertebral fusion cage. The injection temperature was 220°C. The cross-section was quadrilateral, and the side wall was open with a diameter of 1mm. The finished product was tested. , anti-pressure 985 kg, see Table 1 for in vitro degradation experiments.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com