A kind of bone repair material and its application
A technology for bone repair and chondroitin sulfate, applied in the fields of medical biomaterials and medical devices, can solve problems such as poor mechanical properties and strong immunogenicity, and achieve the effects of low storage conditions, convenient storage, and significant bone defect repair effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
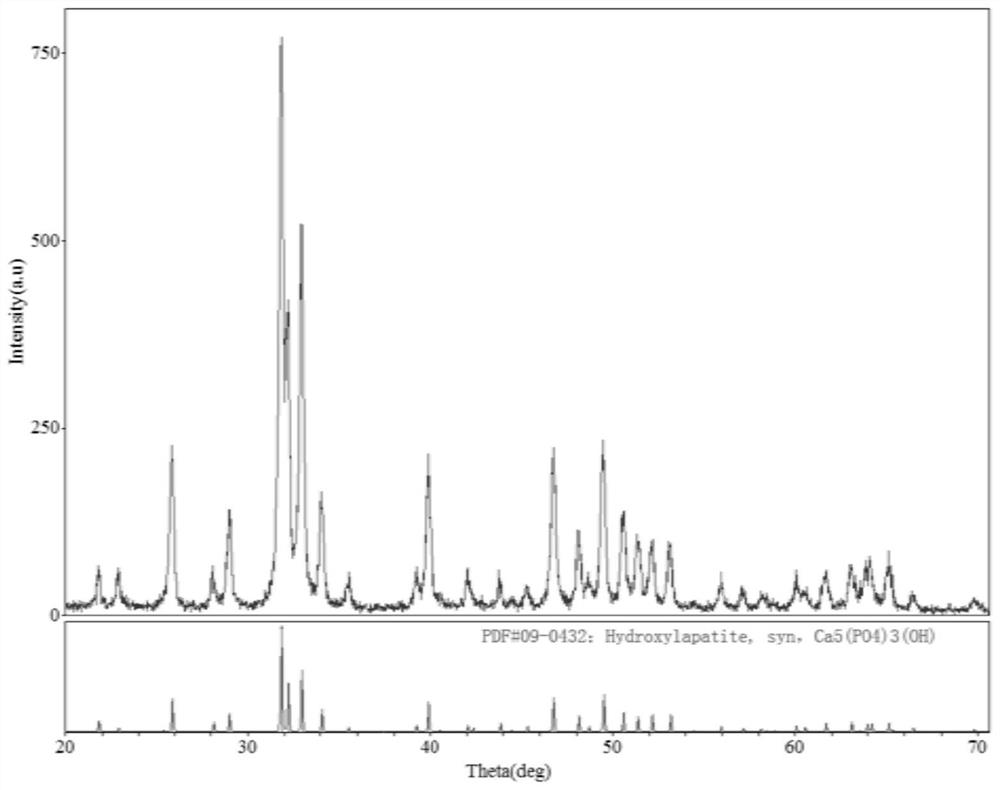
[0048] Example 1: Evaluation of the composition and composition of the bone powder prepared as a raw material
[0049] Choose bone sample (ionic skeleton) via a strong base, strong oxidizing agent, hot water soaked, degreasing, deprotein, decell, dehydrated, calcined by high temperature, inactivating possible viruses, abrasive, sieve, regulate pH To neutral, get natural bone powder.
[0050] 1) The varying skeleton is derived from a healthy cattle under the age of 3 years old, cutting the bone block with no more than 2 cm, rinsing the blood on the bone block.
[0051] 2) The alkalization purpose is to degreasing, decellular, formulated with an alkali: 1M NaOH solution.
[0052] One alkalization: the ratio of the mass volume of the bone block and the alkali solution is 1: 2, soaked for 24 h.
[0053] Induction: peeling the impurities on the bone block.
[0054] Secondary alkalization: the ratio of the mass volume of the bone block and the alkali solution is 1: 2, soaked for 24 h.
...
Embodiment 2
[0067] Example 2: Preparation of Bone Repair Materials
[0068] 1. Preparation of natural hydroxyapatite: Natural hydroxyapatite (natural bone powder) was prepared according to the method of Example 1;
[0069] 2. Preparation of collagen gel:
[0070] 1) Slowly add 500 ml of chondroitin acetic acid solution to 500 ml of collagen acetic acid swelling liquid, the stirring speed is 15,000-20000 rpm, the stirring time is 1-2 hours, the solution is evacuated, resulting in collagen-sulfate chondroitin Slurry;
[0071] Substitridic acid solution configuration:
[0072] 2-4 grams of sulfuric acid chondroitin was added to a 500 ml concentration of 0.4 to 0.6% acetic acid solution, dissolved under temperature conditions at 0-10 ° C, and filtered.
[0073] Collagen acetic acid swelling liquid configuration:
[0074] 3-5 g of type I collagen was added to a 500 ml concentration of 0.3 0.6% acetic acid solution, mixed with stirring, the stirring speed was 15,000-200,000 rpm, the stirring temper...
Embodiment 3
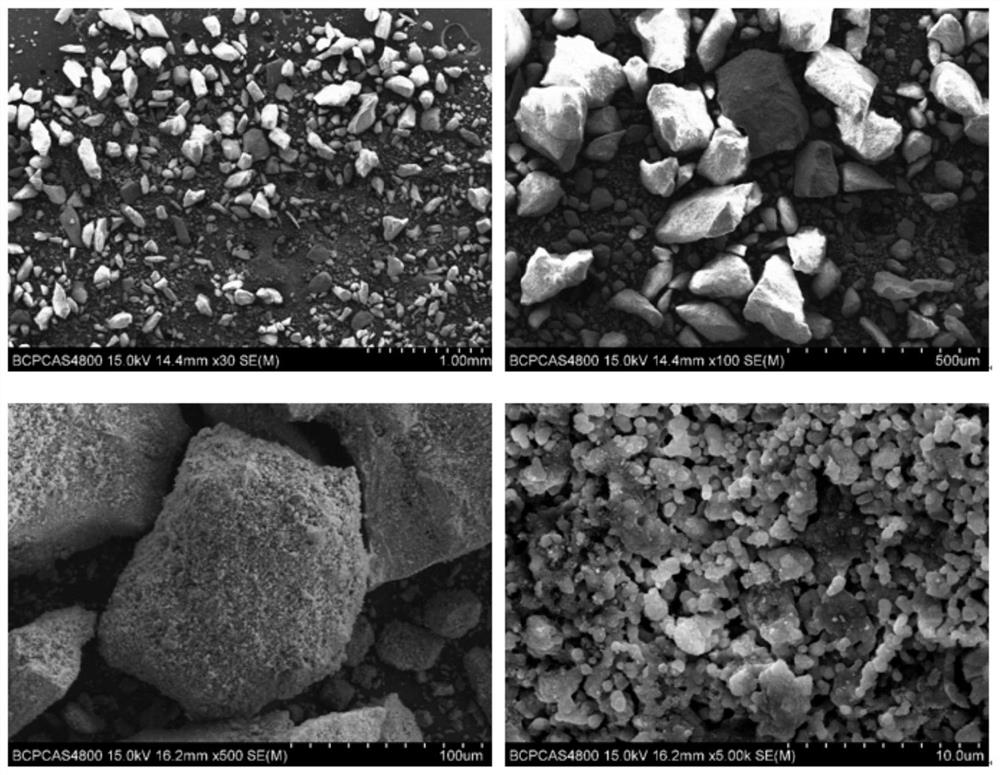
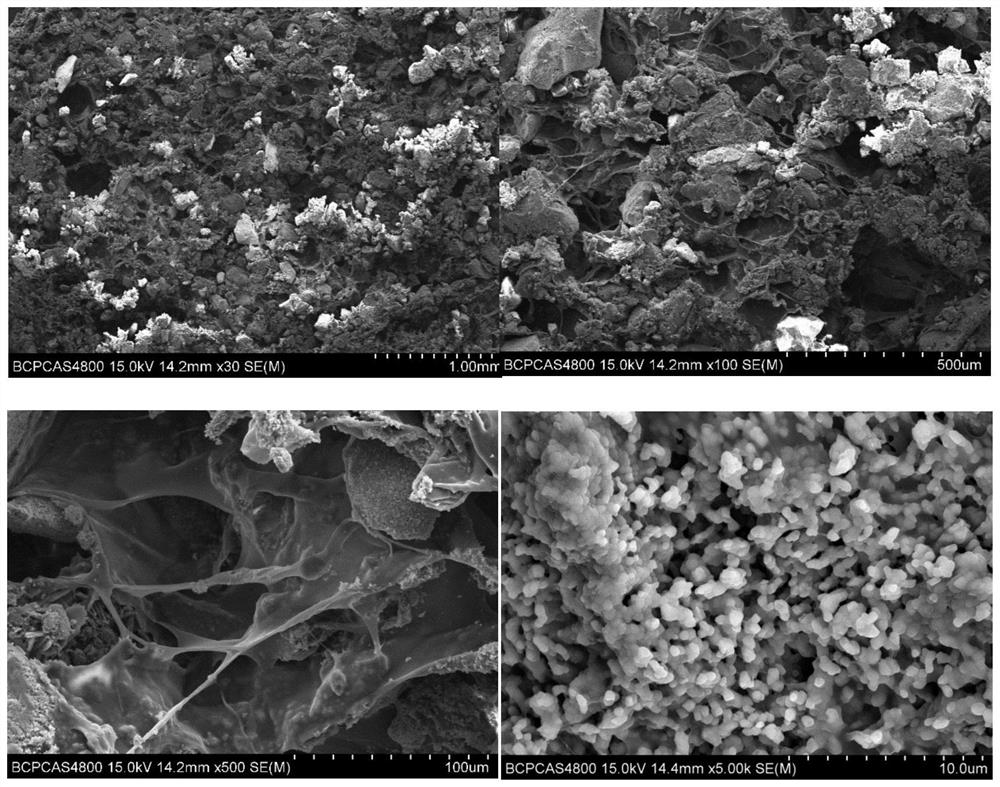
[0087] Example 3: Analysis of the morphology and ingredients of bone repair materials and bone flour
[0088] Example 2 was prepared by a scanning electron microscope (SEM, S4800, HATICHI), and elements distribution analysis were performed by an incidental EDS.
[0089] image 3 For the SEM photo of the bone repair material, it is possible to see that the phosphate particles in the bone repair material are less than 250 μm, and the collagen adhesion is formed, and the collagen is distributed between the collagen and the phosphate particles, the collagen is a smooth film, while the phosphate particles are further The enlargement can be observed that a large amount of 500 nm of hydroxyapatite crystals, and the nanocrystals form a micro-nano-stage hole structure. This structure retains from natural bone microstructure, so that the bone repair material has water absorption and the adsorption of proteins such as growth factor. effect. Further analysis of the bone repair materials such ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com